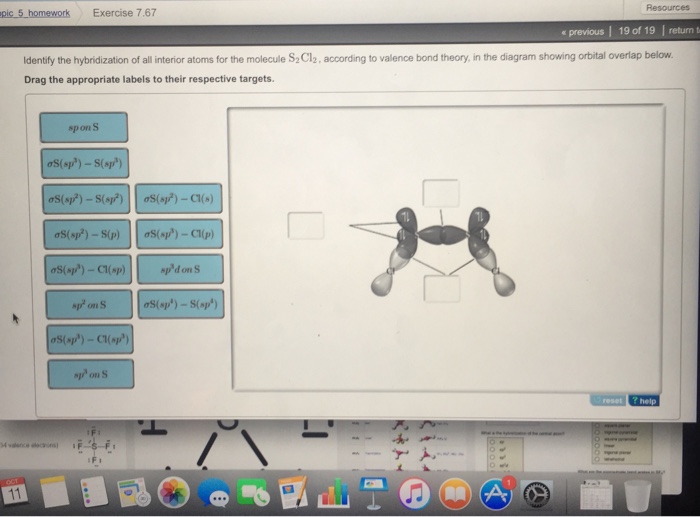
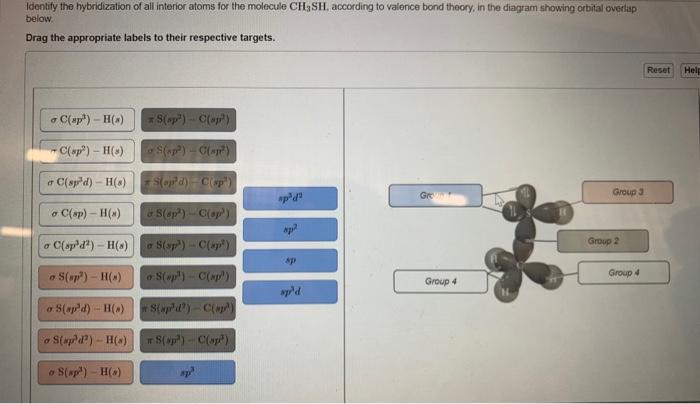
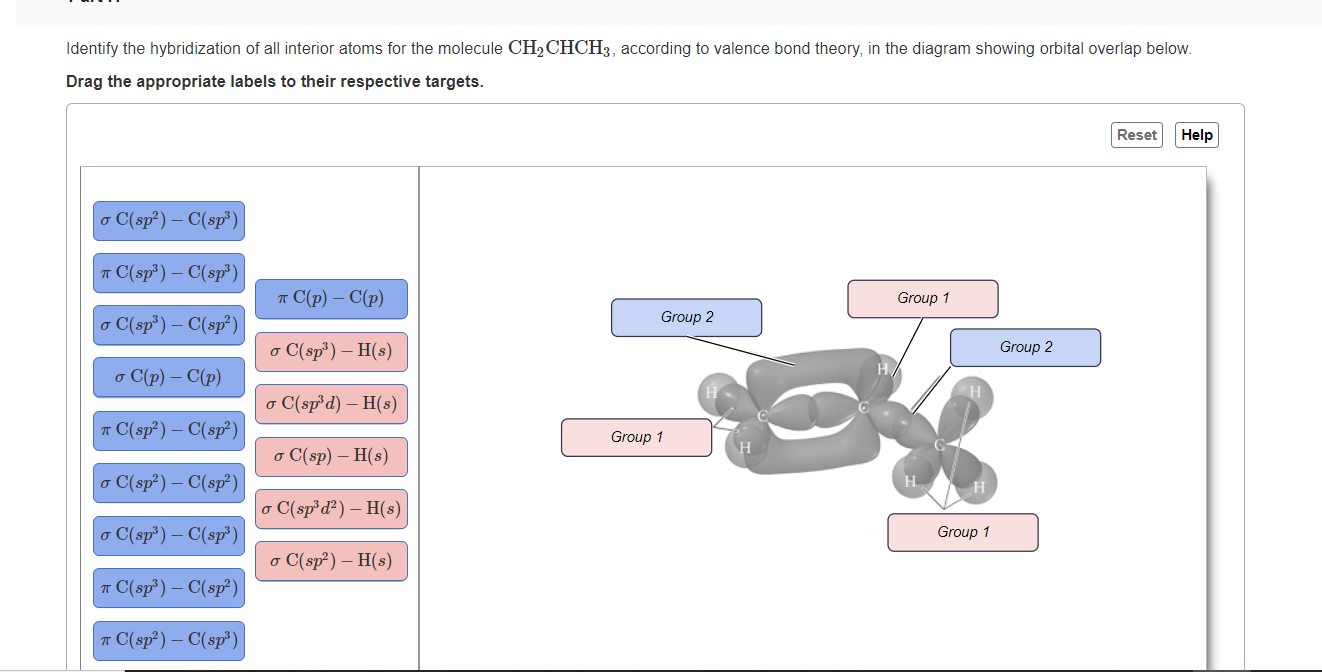
Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com

Draw all nonbonding hybrid orbitals and label the pi and sigma bonds for HCCCH_2OH. | Homework.Study.com

Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com

Draw the Lewis structure for the molecule CH2CHCH3. How many sigma and pi bonds does it contain? | Homework.Study.com
Write the type of hybridisation of each of the carbon atom in the following structures: (i) ch2=c=ch2 (ii) ch3 ch=ch ch3
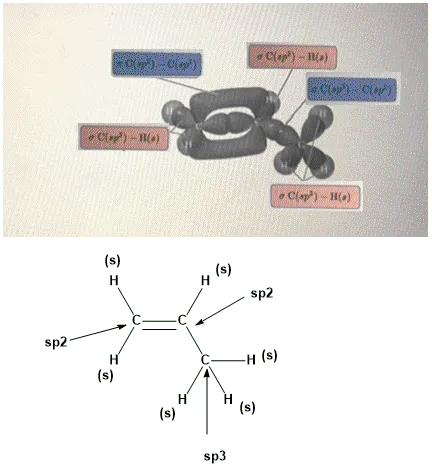
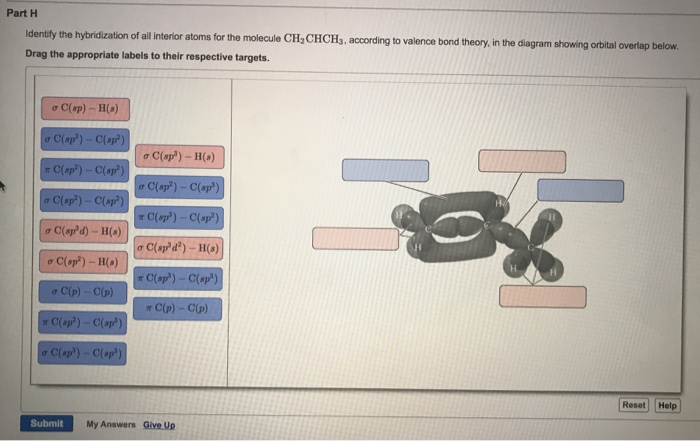
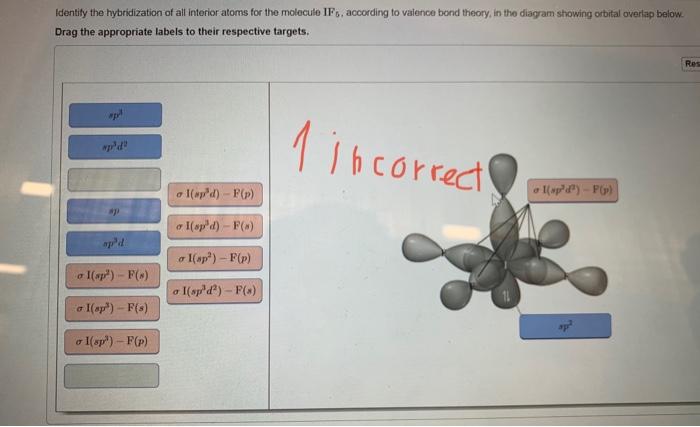
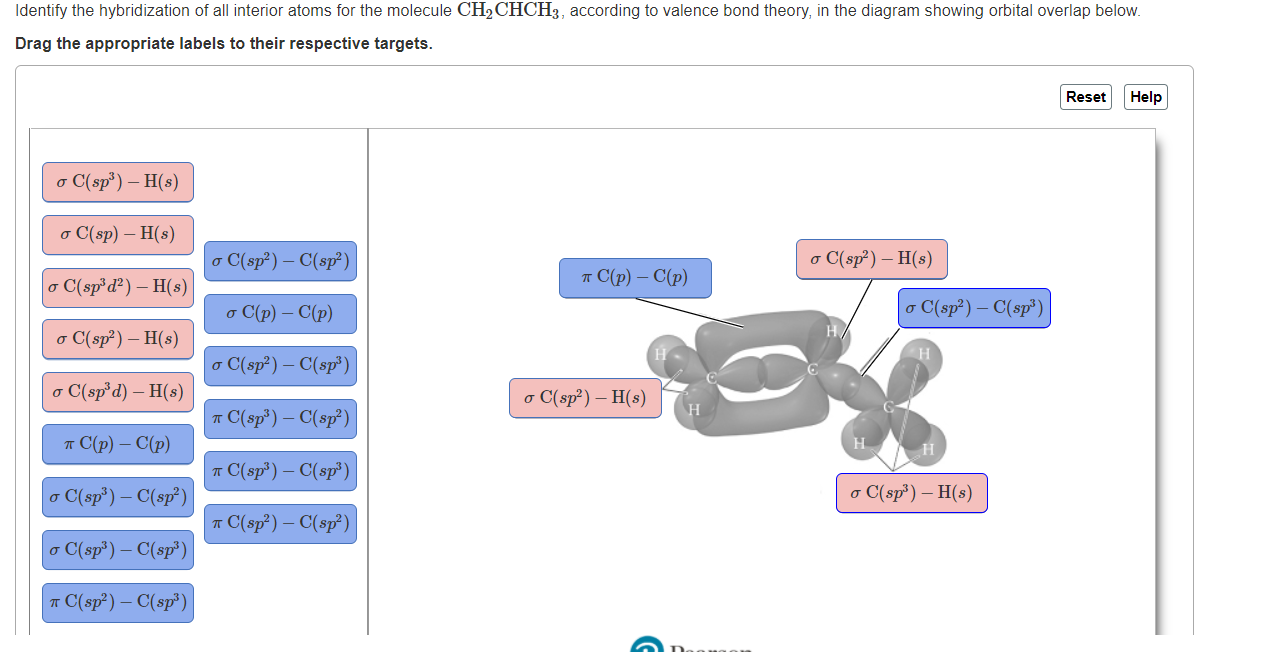
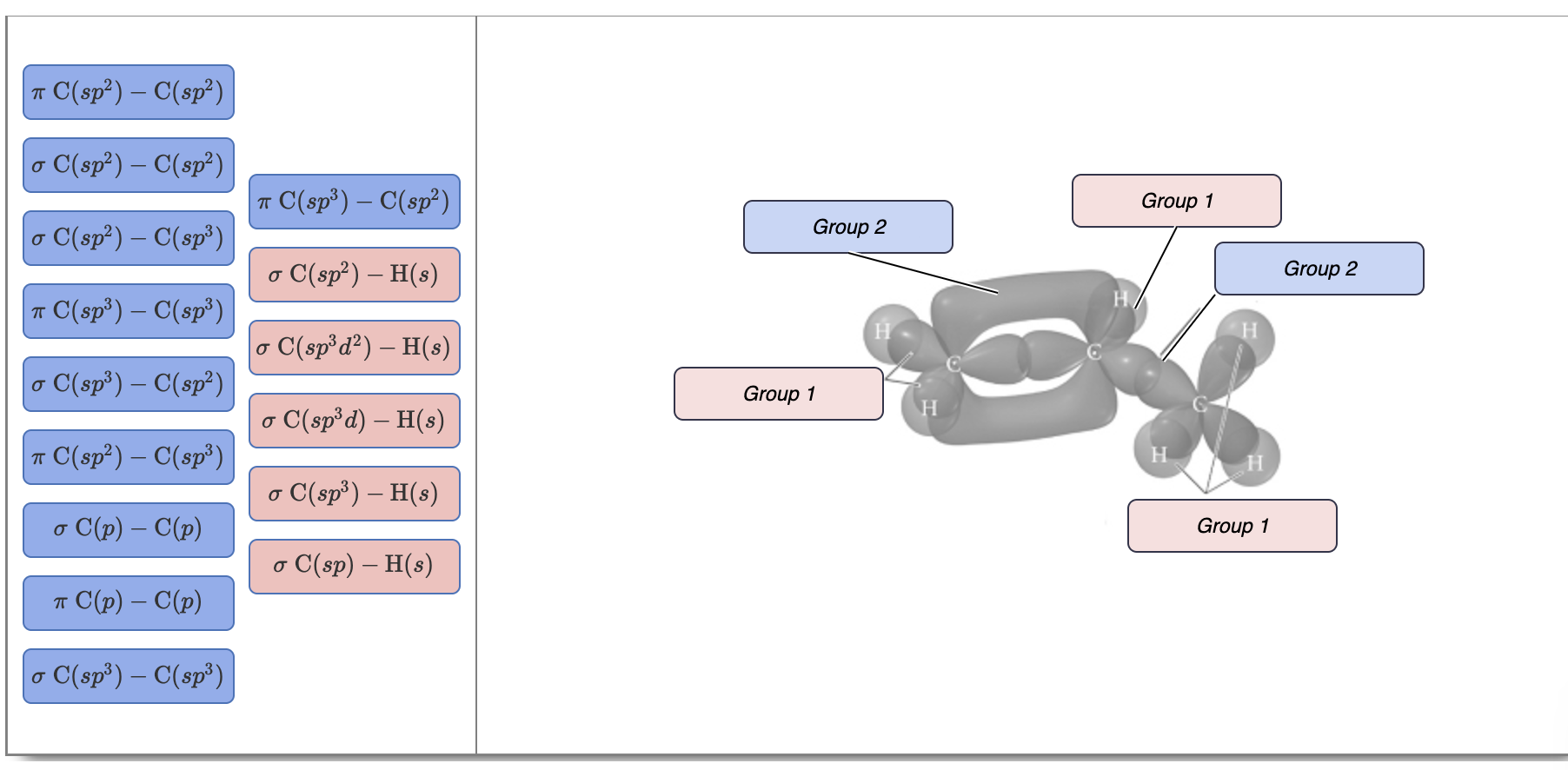
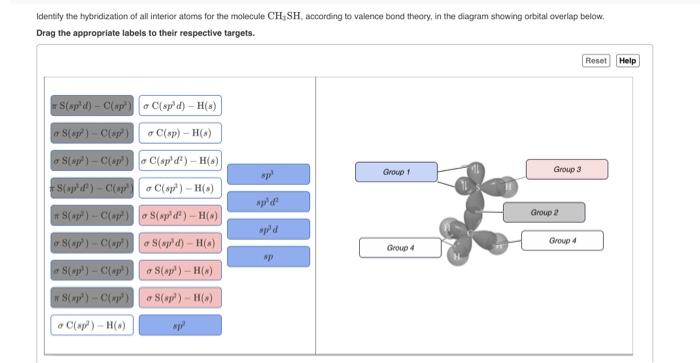
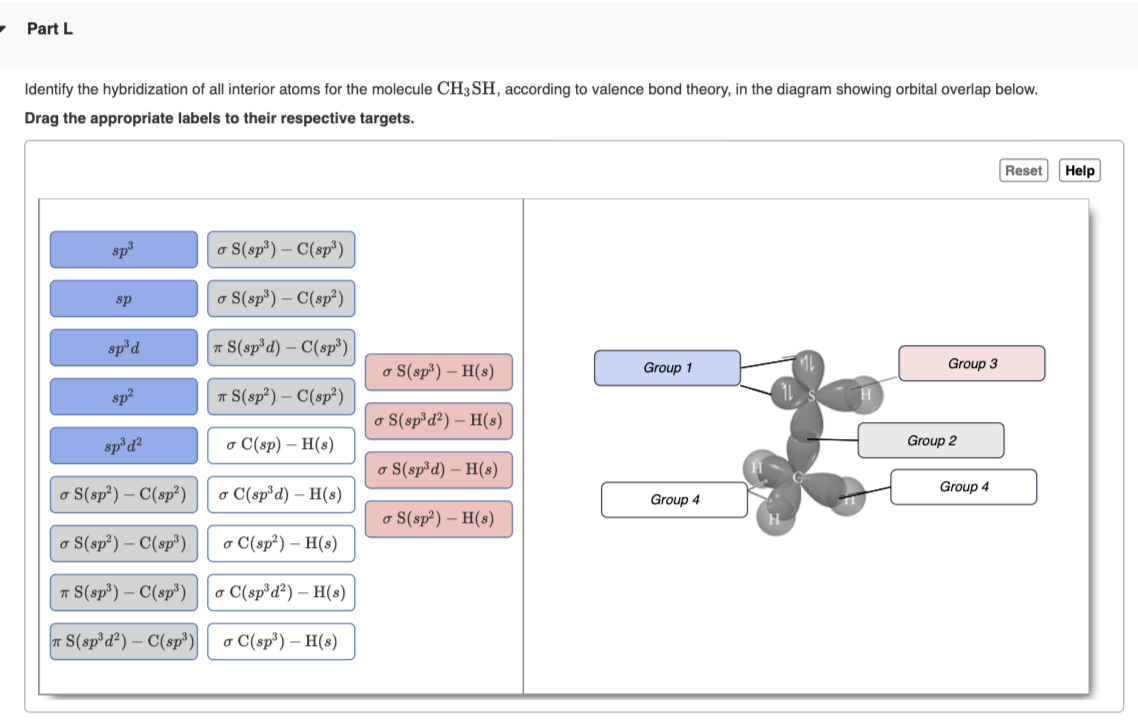
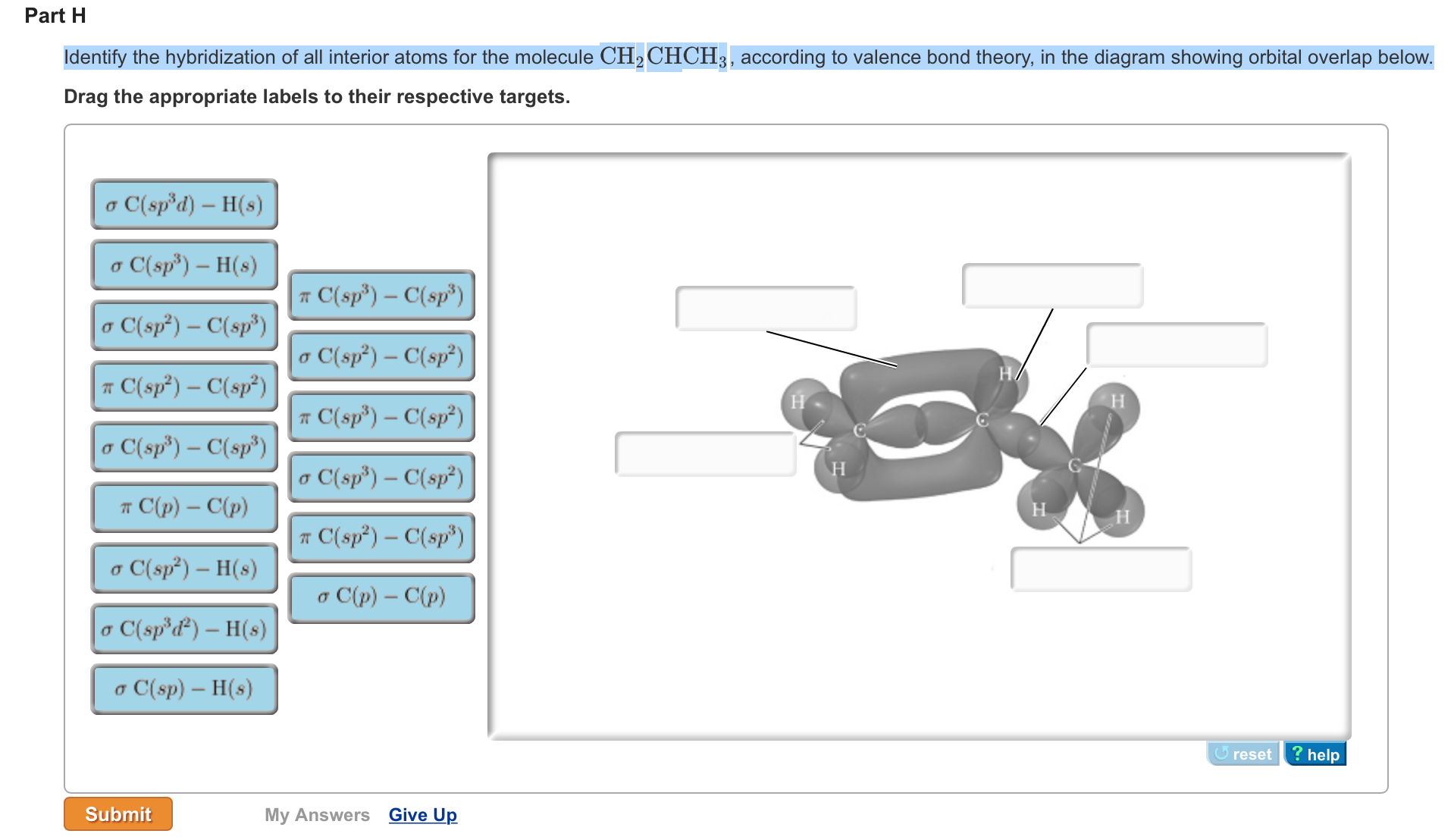
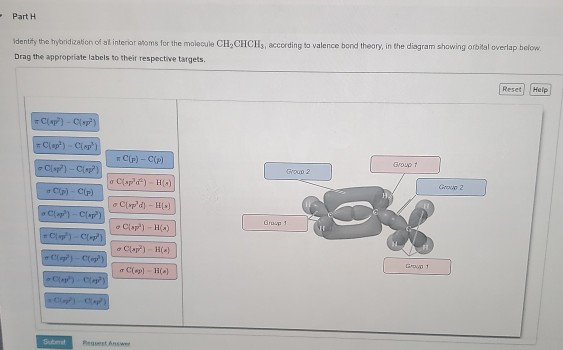
OneClass: Part H Identify the hybridization of all interior atoms for the molecule CH2 CHCHs, accordi...

Explain what is meant by the term "hybridization" in molecular orbital theory and show how the concept can be used to explain the structure and bonding in ethane C2H6, ethene C2H4, and

Describe the molecular geometry around each carbon atom in CH2CHCH3 using VSEPR theory. | Homework.Study.com
University of Thi-Qar … College Of Science ……….. Department of Chemistry Organic Chemistry Second Stage Lecture 1 ( B
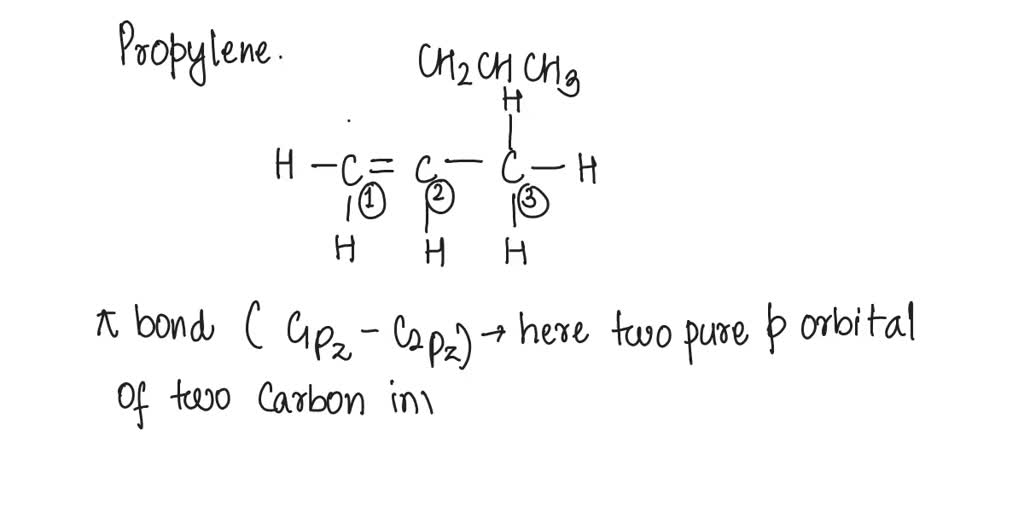
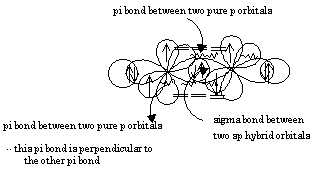
SOLVED: What atomic or hybrid orbitals make up the π bond between C1 and C2 in propylene, CH3(CHCH3)? (C1 is the first carbon in the formula as written: orbital on C1 orbital
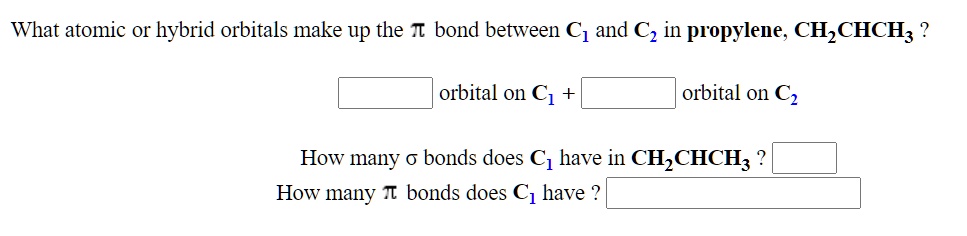
SOLVED: What atomic or hybrid orbitals make up the π bond between C1 and C2 in propylene, CH2CHCH3? π orbital on C1 π orbital on C2 How many bonds does C1 have