
In Cu-ammonia complex, the state of hybridisation of { Cu }^{ 2+ } is:{ sp }^{ 3 }{ d }^{ 3 }sds{ p }^{ 2 }{ sp }^{ 2 }f

Explain the Geometry of Cu(Nh3)4 2+ on the Basis of Hybridisation At. No. Cu = 29 - Chemistry | Shaalaa.com
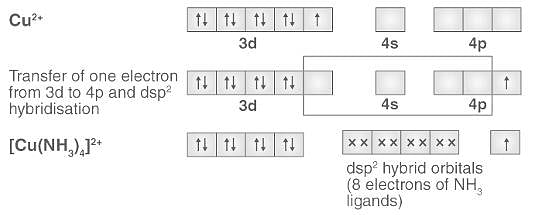
Cuprammonium ion has ______ shape.a)Octahedralb)Tetrahedralc)Trigonald)Square planarCorrect answer is option 'D'. Can you explain this answer? - EduRev Chemistry Question
![PDF) Notizen: E.P.R. determination of the hybridisation and the H-N-H bond angle of ammonia in [Cu(NH3)4][PtCl4] PDF) Notizen: E.P.R. determination of the hybridisation and the H-N-H bond angle of ammonia in [Cu(NH3)4][PtCl4]](https://i1.rgstatic.net/publication/276530089_Notizen_EPR_determination_of_the_hybridisation_and_the_H-N-H_bond_angle_of_ammonia_in_CuNH34PtCl4/links/595bee96458515117741bdc3/largepreview.png)
PDF) Notizen: E.P.R. determination of the hybridisation and the H-N-H bond angle of ammonia in [Cu(NH3)4][PtCl4]
![Cu(NH3)4]2+ এর সংকরায়ন। Hybridization of [Cu(Nh3)4]2+। @N00R007 #chemistry #hybridization - YouTube Cu(NH3)4]2+ এর সংকরায়ন। Hybridization of [Cu(Nh3)4]2+। @N00R007 #chemistry #hybridization - YouTube](https://i.ytimg.com/vi/z0R8tyAjAvY/maxresdefault.jpg)
Cu(NH3)4]2+ এর সংকরায়ন। Hybridization of [Cu(Nh3)4]2+। @N00R007 #chemistry #hybridization - YouTube
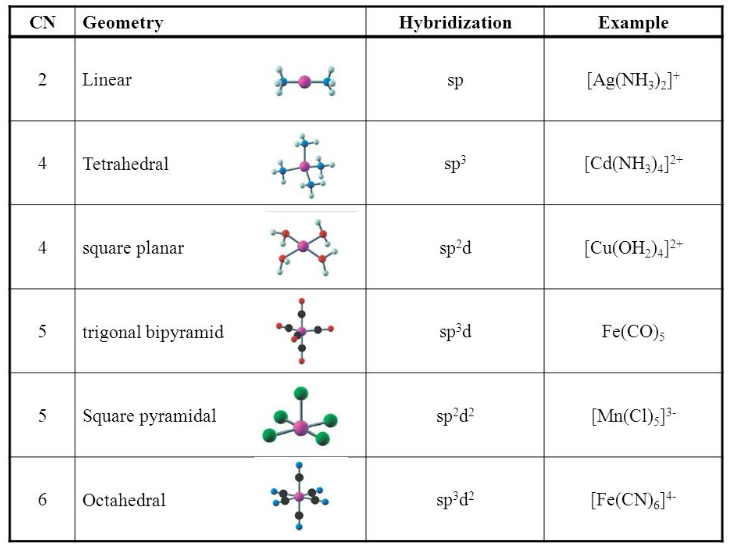
What hybridization is generally utilized by the central atom in a square planar molecule? - CBSE Tuts
![SOLVED: Which is the correct statement(s)? A. [Ag(NH3)2]+ is linear with sp hybridized Ag+ ions. B. NiCl4^2-, CrO4^2-, and MnO4^- have tetrahedral geometry. C. [Cu(NH3)4]^2+, [Pt(NH3)4]^2+, and [Ni(CN)4]^2- have dsp^2 hybridization of SOLVED: Which is the correct statement(s)? A. [Ag(NH3)2]+ is linear with sp hybridized Ag+ ions. B. NiCl4^2-, CrO4^2-, and MnO4^- have tetrahedral geometry. C. [Cu(NH3)4]^2+, [Pt(NH3)4]^2+, and [Ni(CN)4]^2- have dsp^2 hybridization of](https://cdn.numerade.com/project-universal/previews/1561ff27-3e90-4193-9ac2-8cbafea01b2c.gif)

![Hybridization and magnetic moment of `[Cu(NH_(3))_(4)]^(2+)` and `[Mn(CN)_(6)^(3-)` ions - YouTube Hybridization and magnetic moment of `[Cu(NH_(3))_(4)]^(2+)` and `[Mn(CN)_(6)^(3-)` ions - YouTube](https://i.ytimg.com/vi/owlgbfjVOzc/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLBv6TqxUZtnijDOJn7QLMuUGig2-A)

![Hybridization in [Cu(NH3)4]2+ ion - YouTube Hybridization in [Cu(NH3)4]2+ ion - YouTube](https://i.ytimg.com/vi/mY8bvkr94QQ/sddefault.jpg)
![Solved] what is ths hybridization of cu in cu[(nh3)4]2+ - Brainly.in Solved] what is ths hybridization of cu in cu[(nh3)4]2+ - Brainly.in](https://hi-static.z-dn.net/files/de4/49aeb4fd2144e40e1aba99873c3f2a73.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)
![Cu(NH(3))(4)]^(2+) is a square planar complex.The number of unpaired Cu(NH(3))(4)]^(2+) is a square planar complex.The number of unpaired](https://static.doubtnut.com/ss/web-overlay-thumb/761702.webp)

![Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRK_10Y_SP_HSC_CHE_JLY_18_E02_001_S01.png)