
SOLVED: Consider the molecule with the connectivity H-N-N-H: The hybridization at each nitrogen atom is The double bond between the nitrogen atoms is made by the overlap of two hybridized orbitals, producing (

Karthik0000 assignment - 36 Determinetheelectrongeometry moleculargeometry andidealizedbondanglesforeachof thefollowingmolecules.Inwhichcasesdoyouexpect | Course Hero
Free energy change for the formation of *N2H2 (i.e. step: *N2H + (H⁺ +... | Download Scientific Diagram

Experimental evidence suggests that the nitrogen atom in ammonia, NH3, has four identical orbitals in the shape of a pyramid or tetrahedron. Draw an energy-level diagram to show the formation of these

Solved) - 9.62 The nitrogen atoms in N 2 participate in multiple bonding,... - (1 Answer) | Transtutors

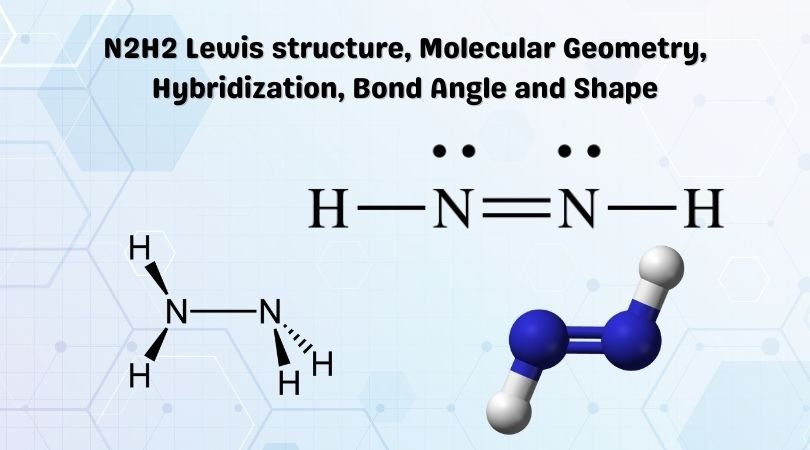
a. Determine the electron geometry of N_2H_2 (skeletal structure HNNH). Indicate the geometry about one central atom. b. Determine the molecular geometry of N_2H_2 (skeletal structure HNNH). Indicate the geometry about one
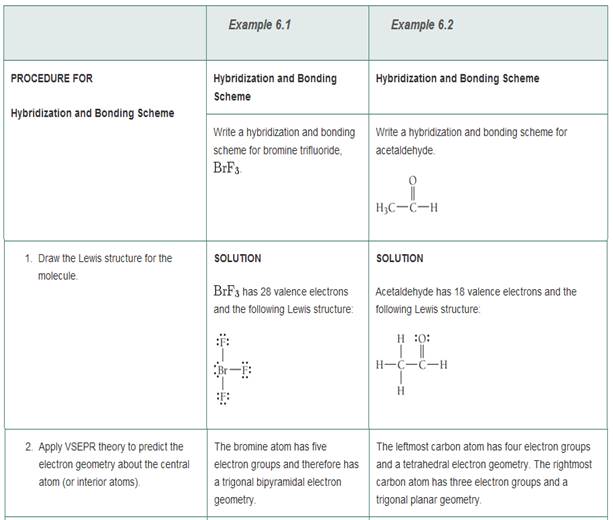
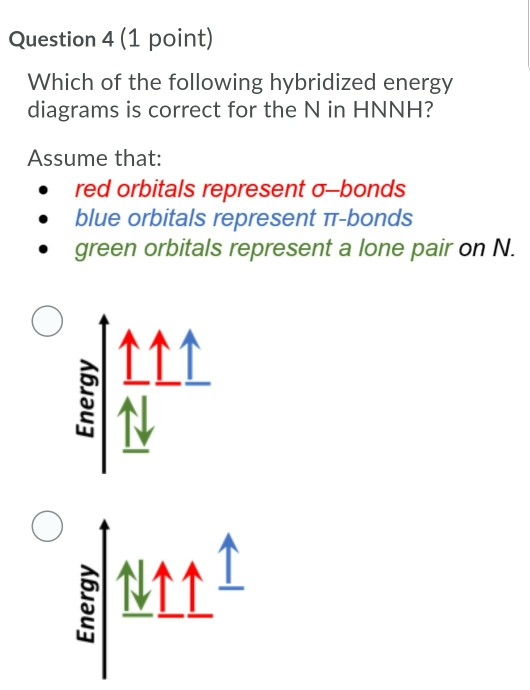
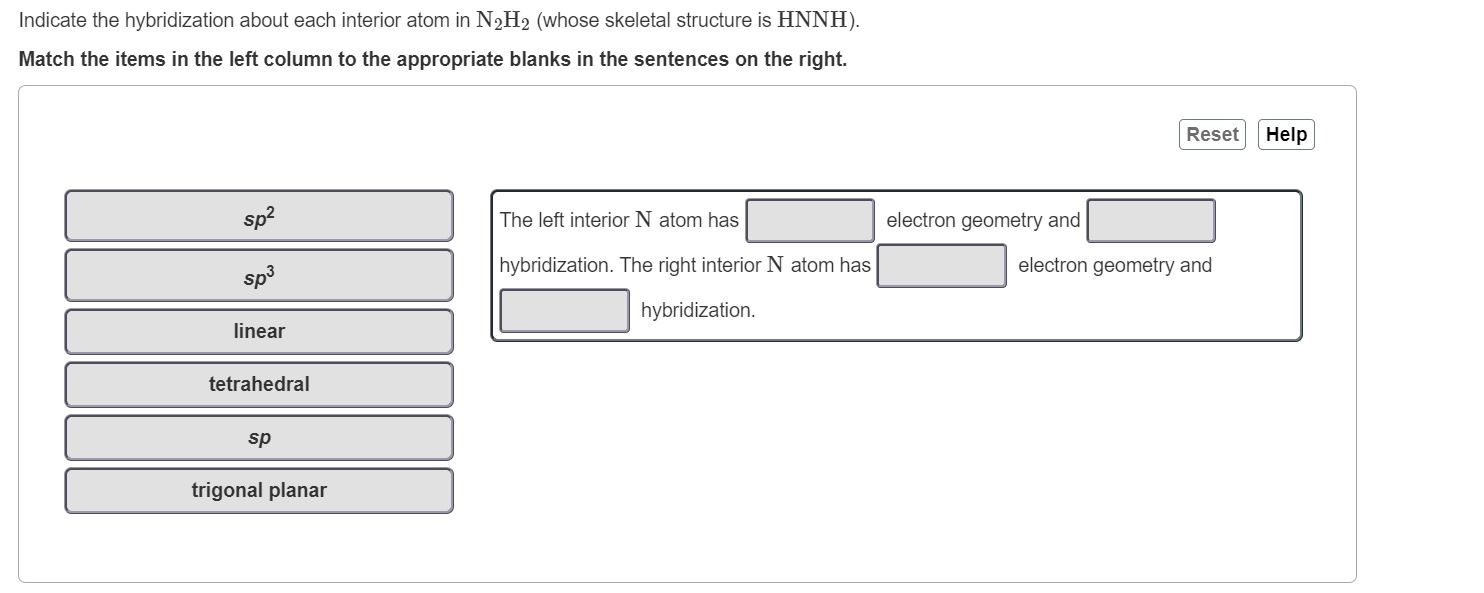
Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all
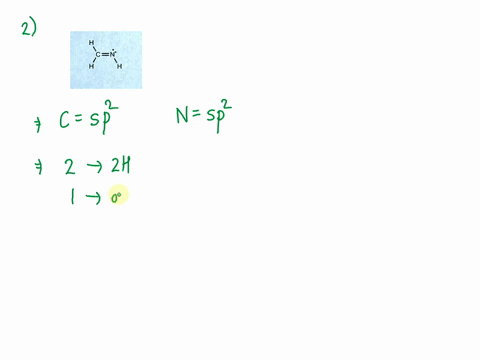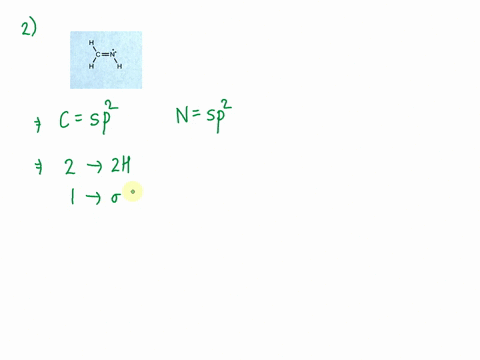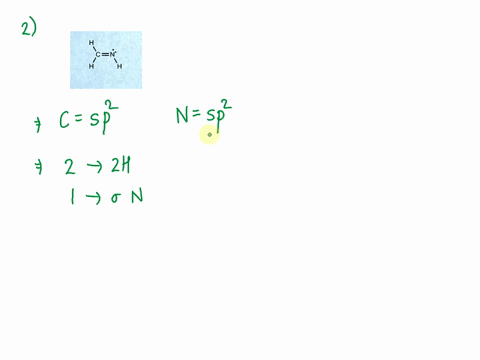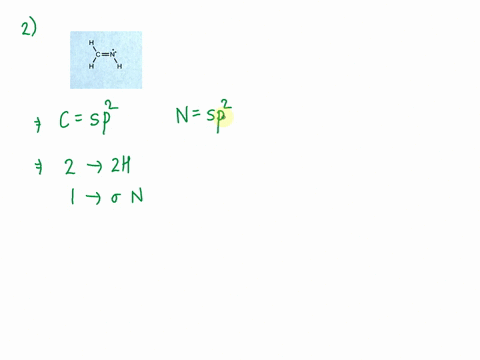
SOLVED: Consider the molecule with the connectivity H-N-N-H: The hybridization at each nitrogen atom is The double bond between the nitrogen atoms is made by the overlap of two hybridized orbitals, producing (

SOLVED: Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation ...

Two structures can be drawn for cyanuric acid as follows.Give the hybridization of the carbon and nitrogen atoms in each structure. | Homework.Study.com
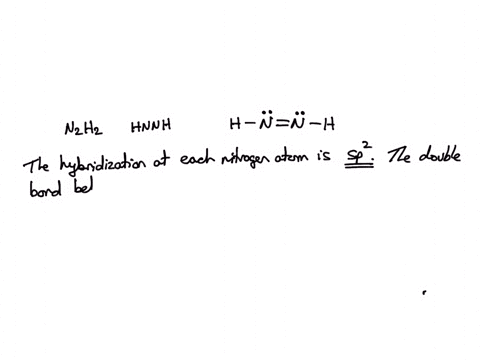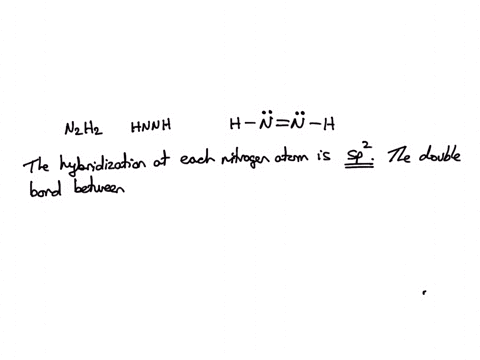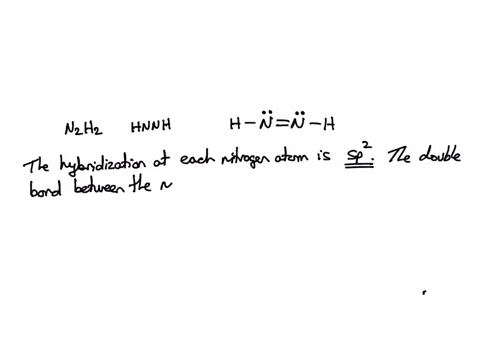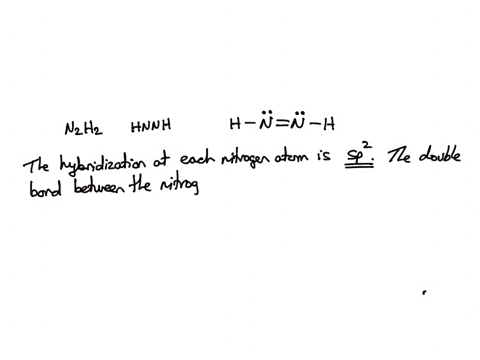









![ANSWERED] Part B Indicate the hybridization about each interior atom - Kunduz ANSWERED] Part B Indicate the hybridization about each interior atom - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20220621022736275335-4634392.jpg)