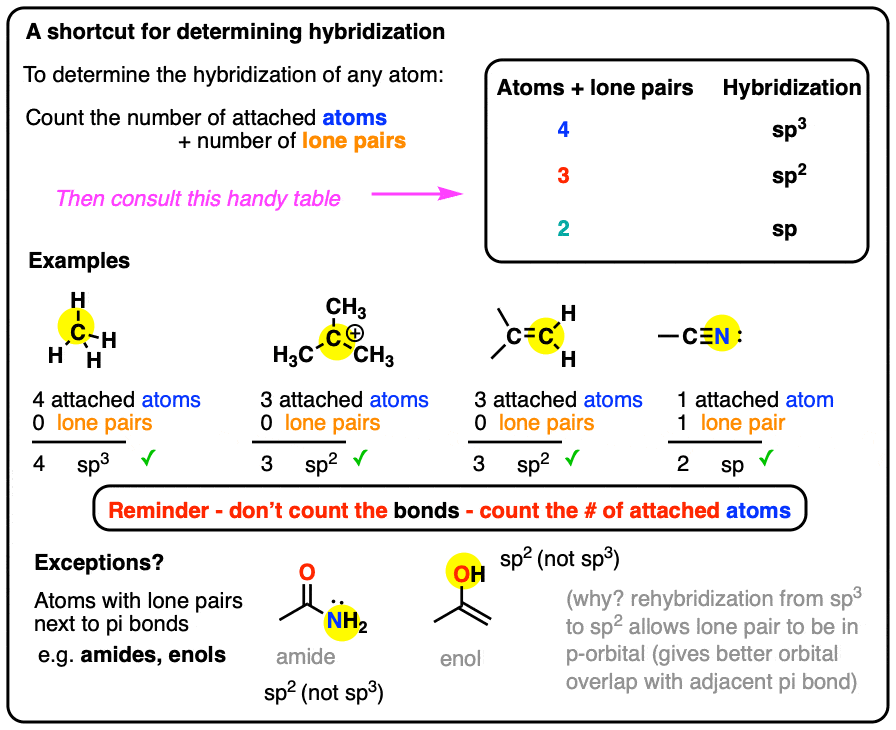
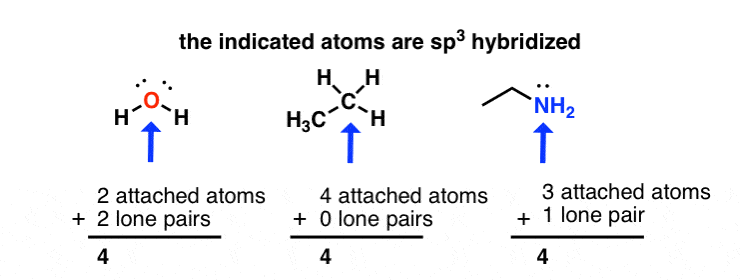
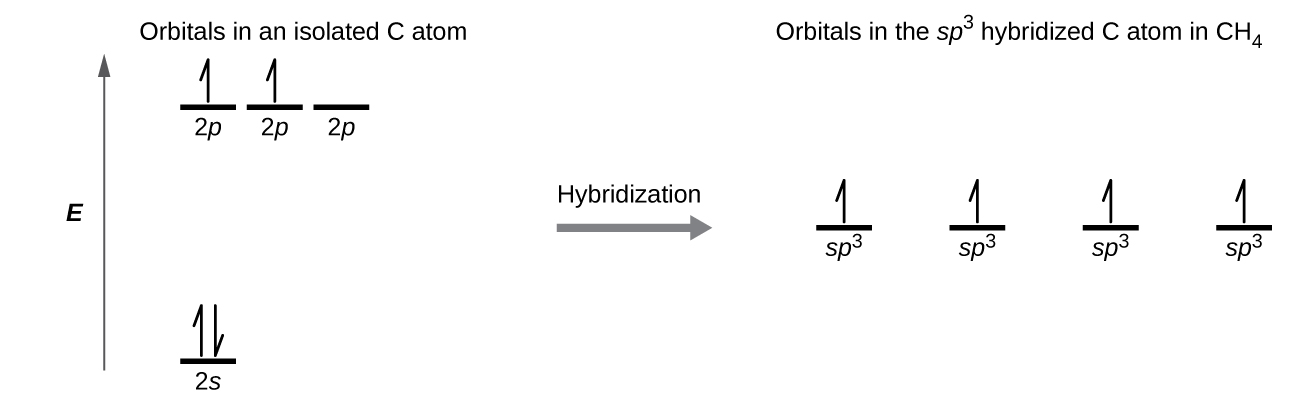
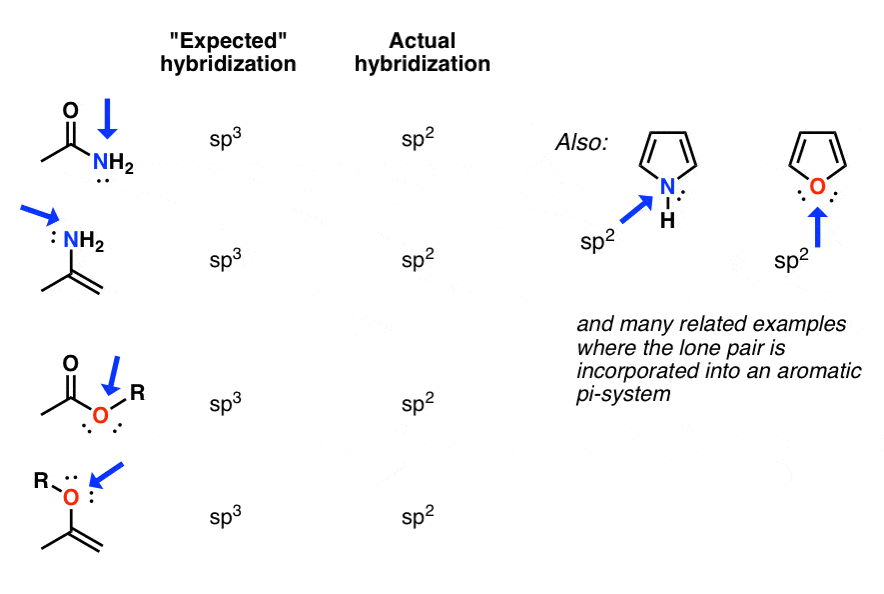
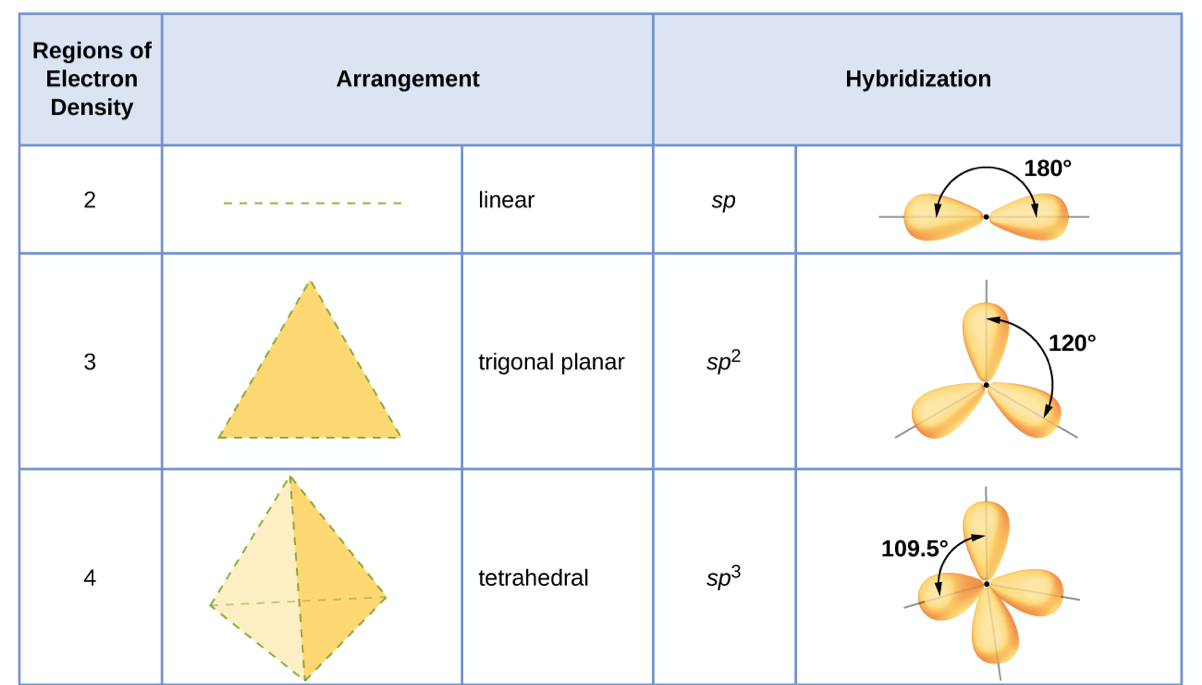
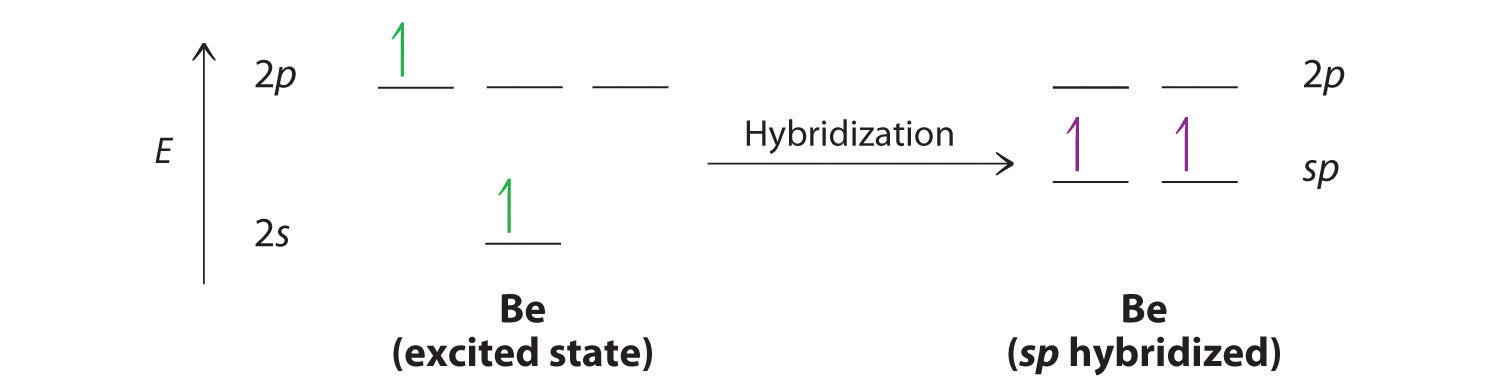
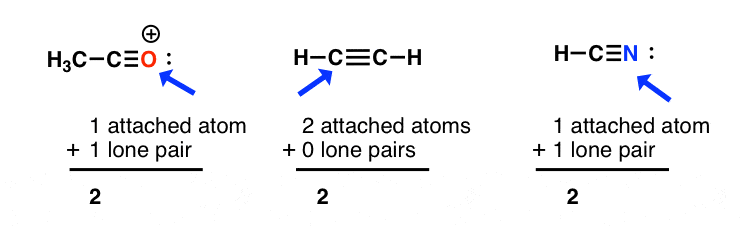
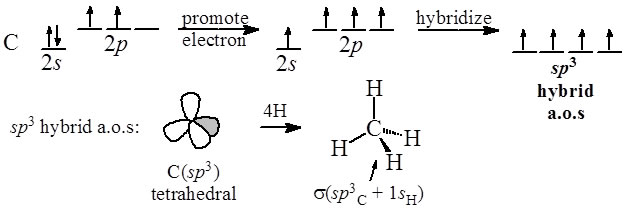
Hybridization of central atoms in the moleculesN(CH3)3 and N(SIH3) respectively are(1) sp2 and sp2(2) sp3 and sp3(3) sp2 and sp34) sp3 and sp2
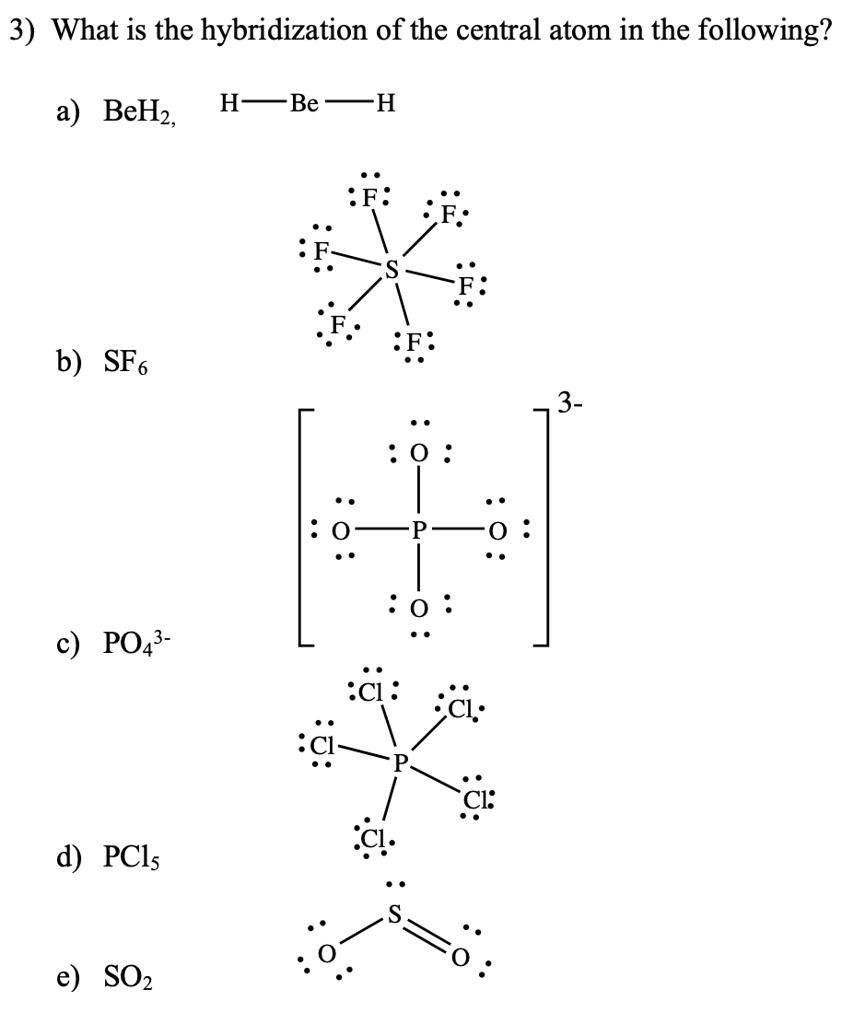
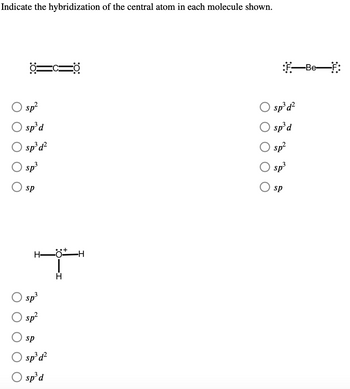
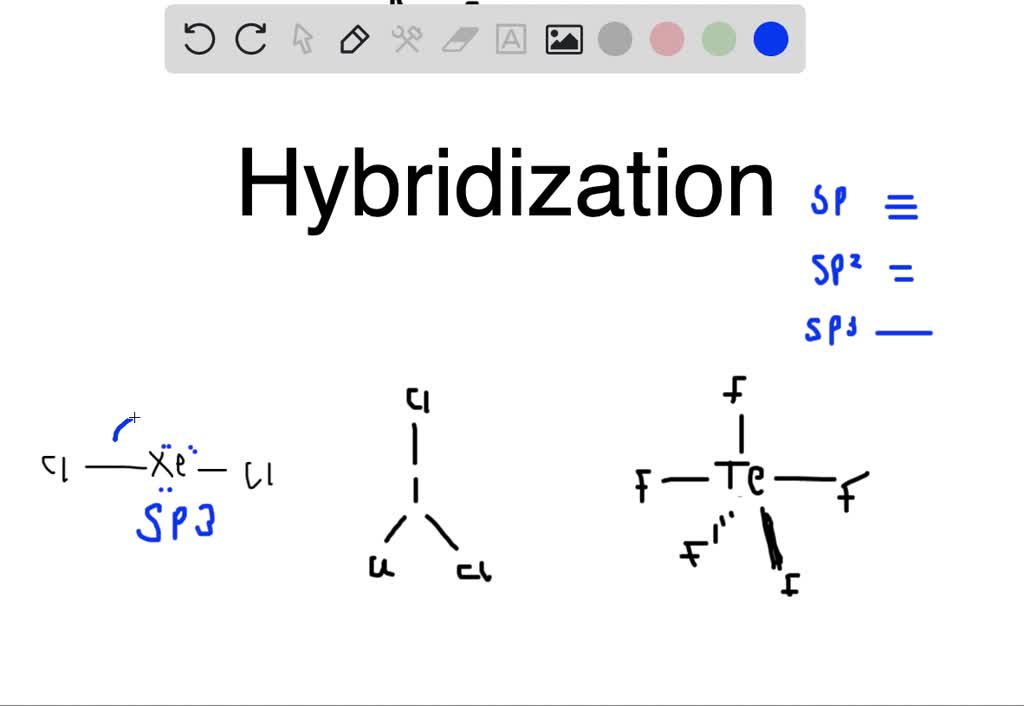
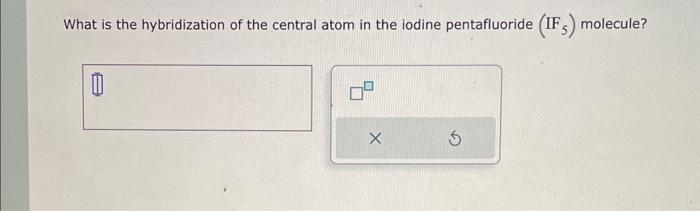
SOLVED: What is the hybridization of the central atom in the following? H2O a) BeH2, b) SF6 c) PO3^- d) PCl5 e) SO2