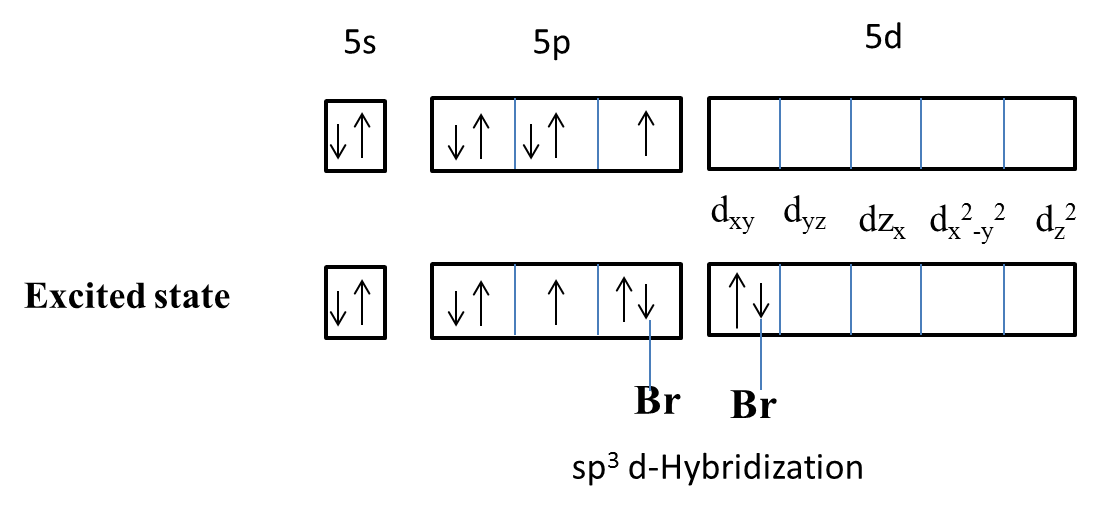
Hybridization of iodine atoms in ICl_(3) (in its stable form, found in solid state) and I_(2)Cl_... - YouTube
What is the hybridisation of iodine in IF7 ? Give its structure. - Sarthaks eConnect | Largest Online Education Community

SOLVED: What is the hybridization of the iodine atom in the Lewis structure shown below? To answer the next three questions, refer to the Lewis structure at the top of page 8.

Write down the Lewis structure of ICl3. Provide the electron pair geometry, molecular geometry, and hybridization of iodine. | Homework.Study.com
![In the structure of \\[I{{F}_{7}}\\] (Iodine heptafluoride) ----------.(A) \\[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\\] orbitals are involved in hybridization(B) Axial bonds are longer than equatorial bonds(C) There are 10 different orthogonal angels(D)The ... In the structure of \\[I{{F}_{7}}\\] (Iodine heptafluoride) ----------.(A) \\[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\\] orbitals are involved in hybridization(B) Axial bonds are longer than equatorial bonds(C) There are 10 different orthogonal angels(D)The ...](https://www.vedantu.com/question-sets/d7f0d18e-1e05-4073-8dfc-eafee1295a3c8458677129858264325.png)
In the structure of \\[I{{F}_{7}}\\] (Iodine heptafluoride) ----------.(A) \\[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\\] orbitals are involved in hybridization(B) Axial bonds are longer than equatorial bonds(C) There are 10 different orthogonal angels(D)The ...