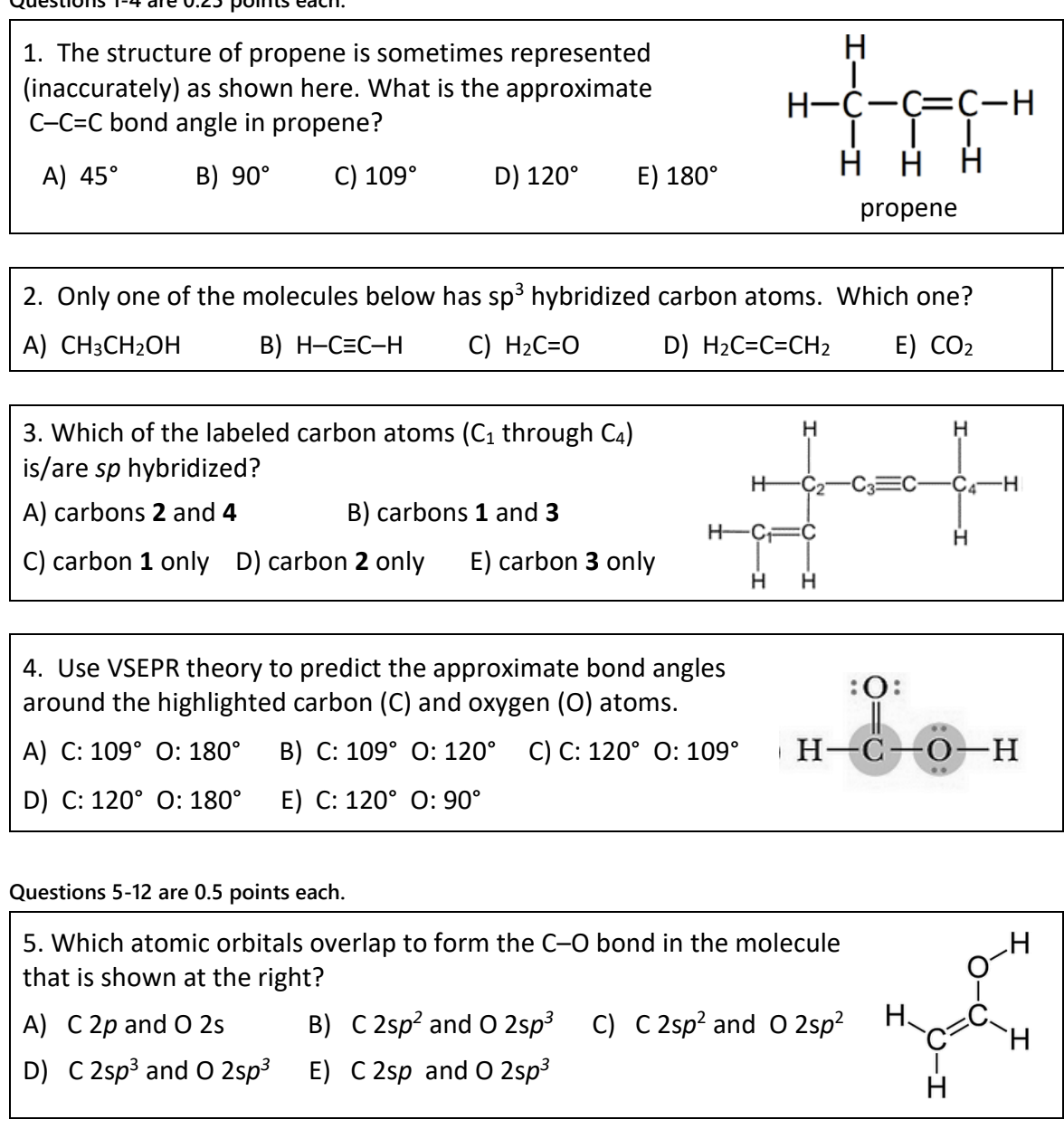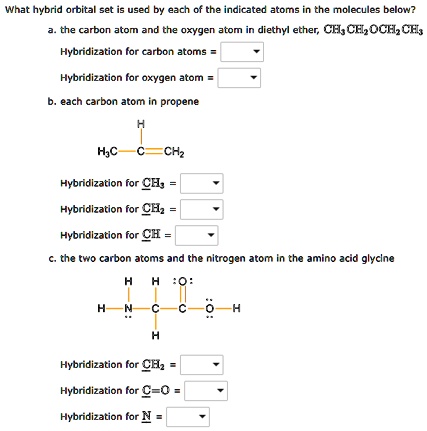
SOLVED: Text: What hybrid orbital set is used by each of the indicated atoms in the molecules below? a. the carbon atom and the oxygen atom in diethyl ether, CH3COCH2CH3 Hybridization for

Orbital Hybridization Practice Problems - Organic Chemistry #stemeducation #stem #chemistry - YouTube
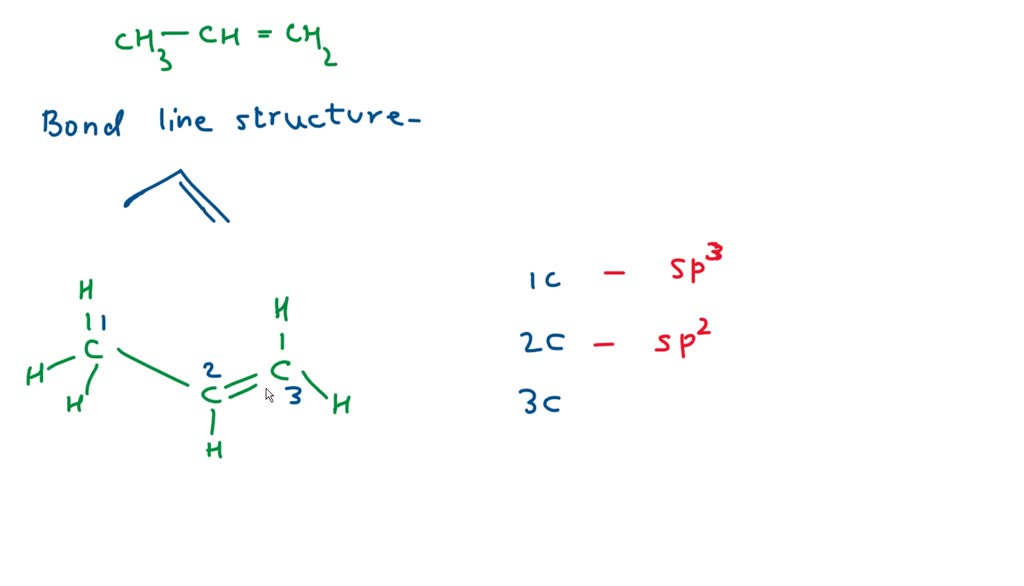
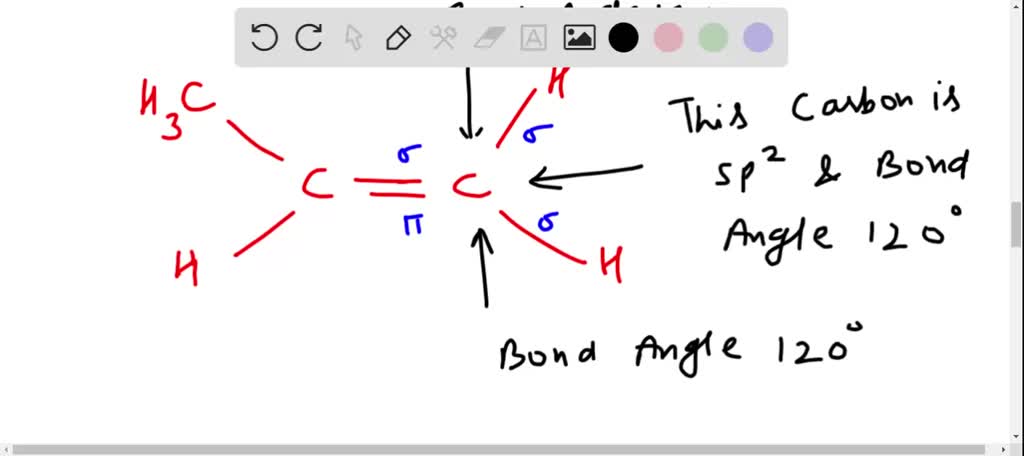
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
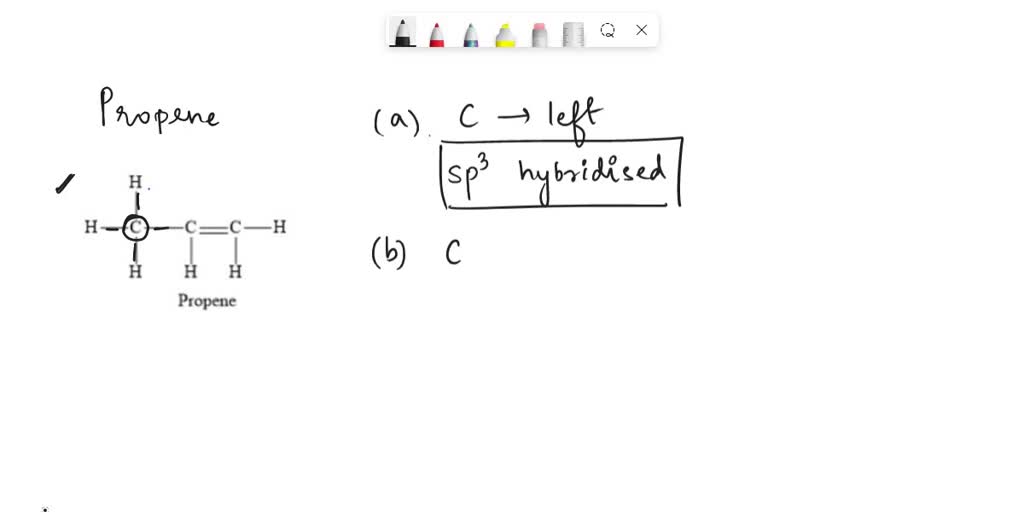
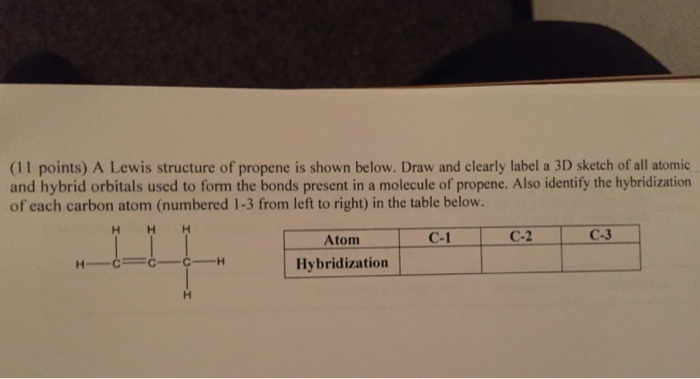
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for
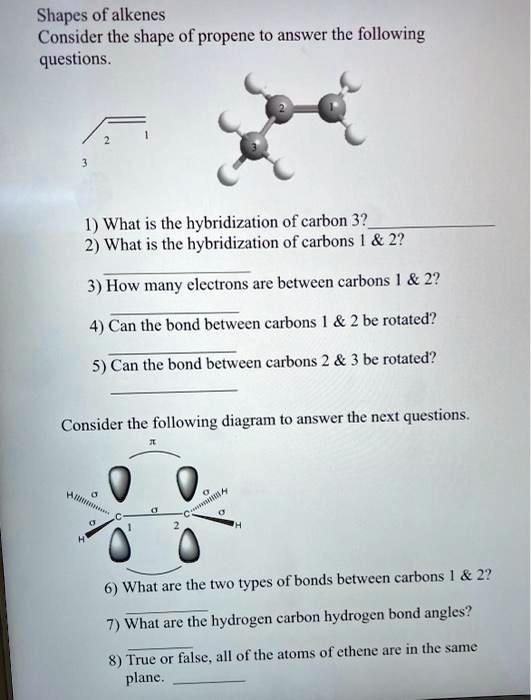
SOLVED: Shapes of alkenes Consider the shape of propene to answer the following questions 1) What is the hybridization of carbon 3? 2) What is the hybridization of carbons | 22 3)

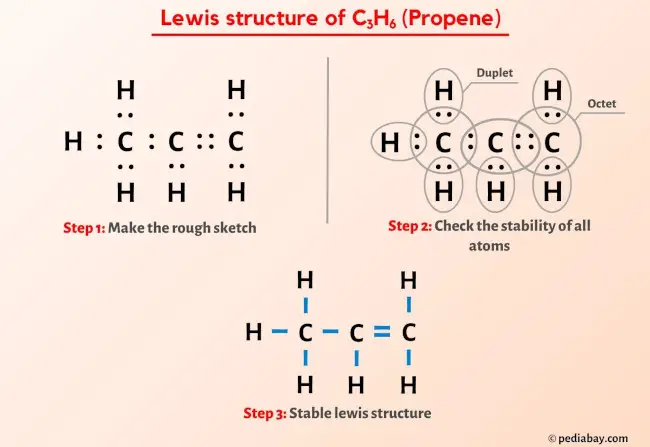
Propene C3H6: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic
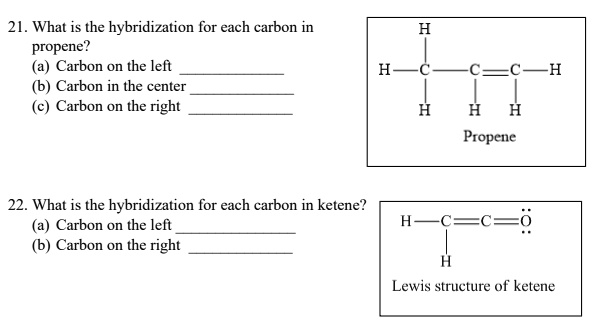
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for
How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora
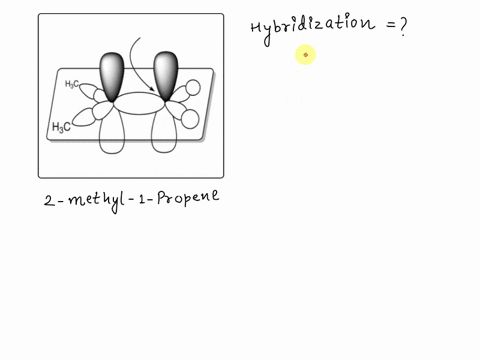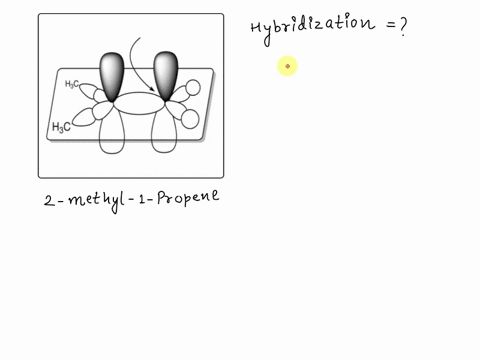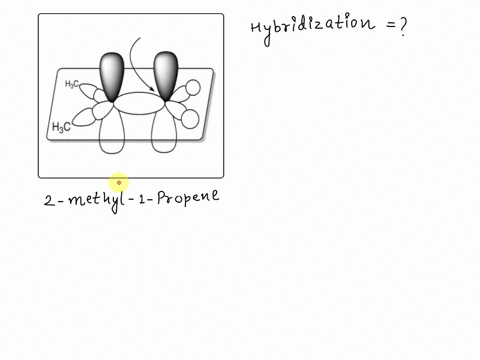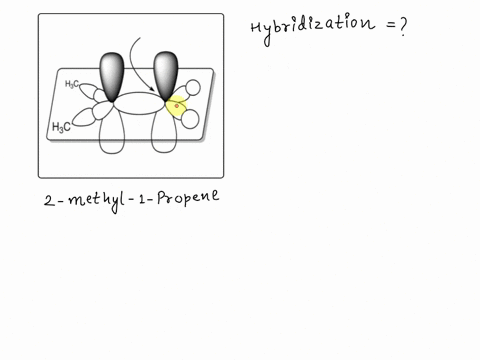
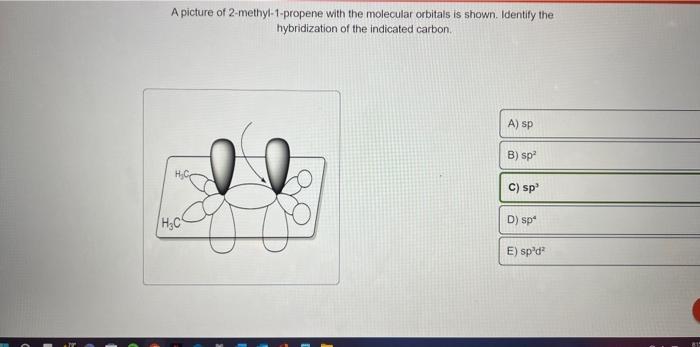
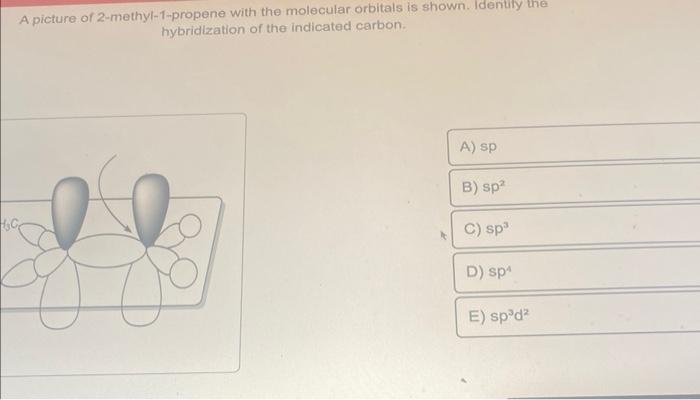
SOLVED: Texts: A picture of 2-methyl-1-propene with the molecular orbitals is shown. Identify the hybridization of the indicated carbon. A) sp B) sp2 C) sp D) sp3 E) sp2