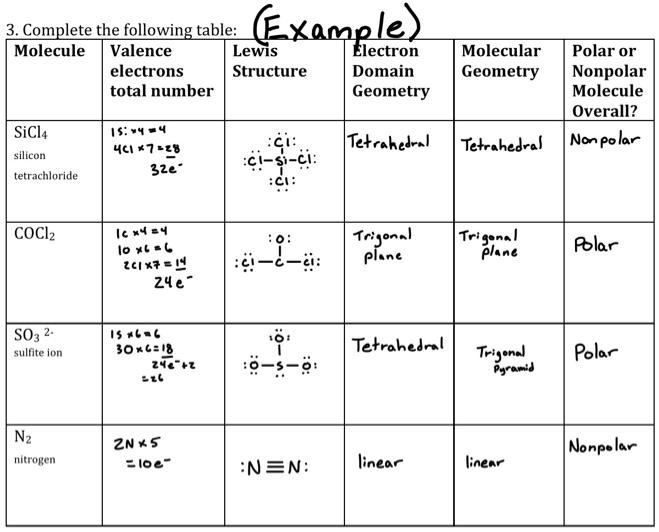
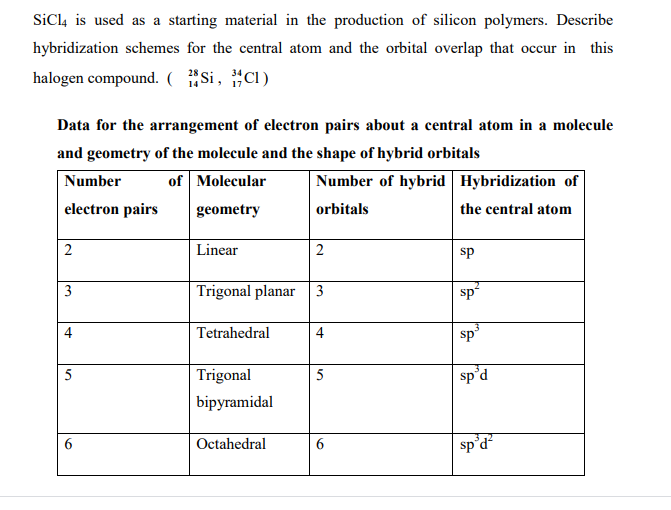
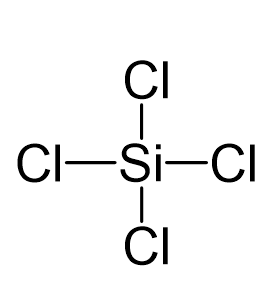
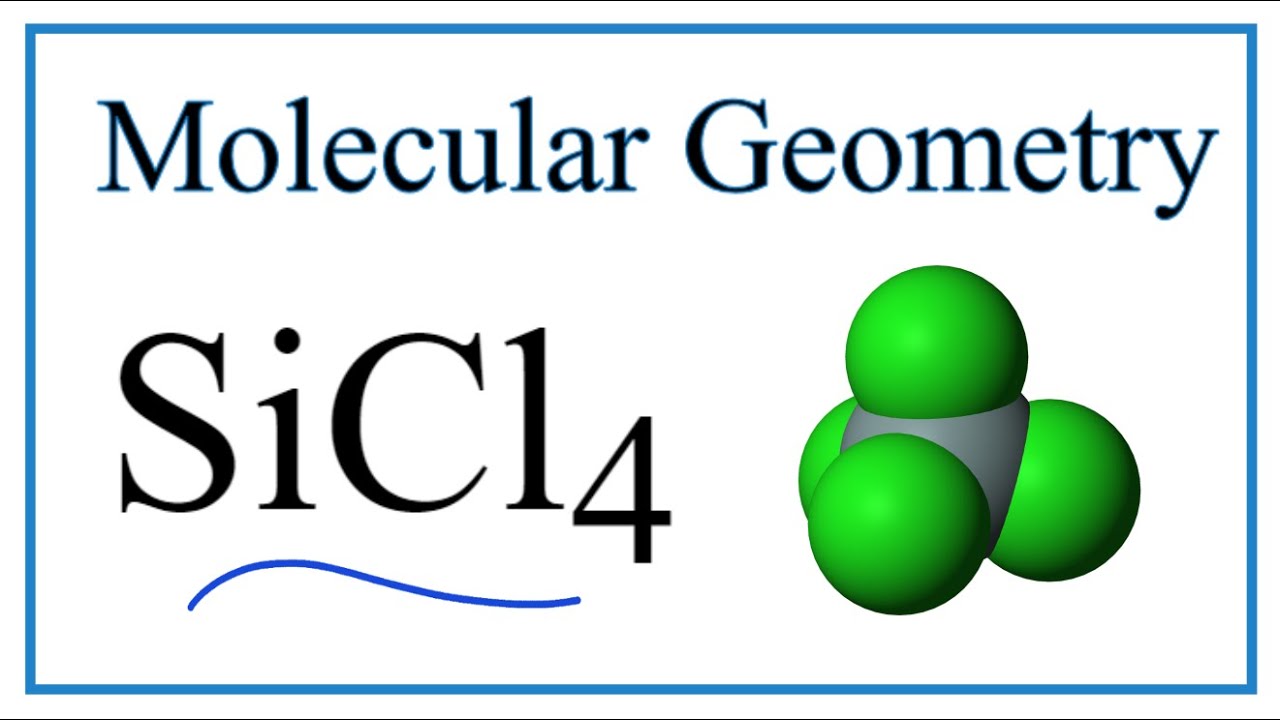
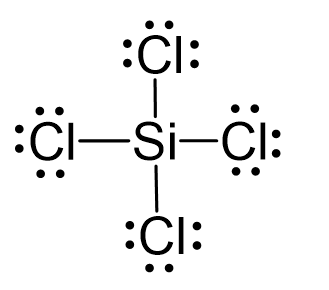
Silicon tetrachloride SiCl4: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure –
In case of hybridization in chemical bonding is only the central atom hybridized or atoms also except central atoms hybridize to form bonds? What is the exact concept of hybridization? - Quora
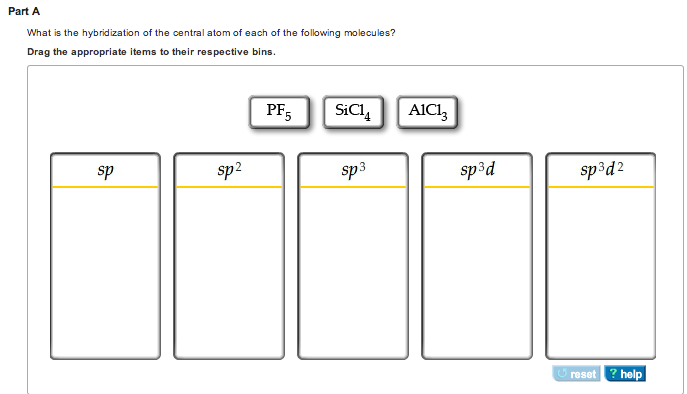
What is the hybridization of the central atom of each of the following molecules? - Home Work Help - Learn CBSE Forum
sp^3 (sp three) Hybridization (Tetrahedral hybridisation). - Sarthaks eConnect | Largest Online Education Community
Q. Which of the following pair has same hybridisation. 1] BF3 NF3 2] SF4 SiCl4 3] ClO4 ClO2 4] CO2 SiO2
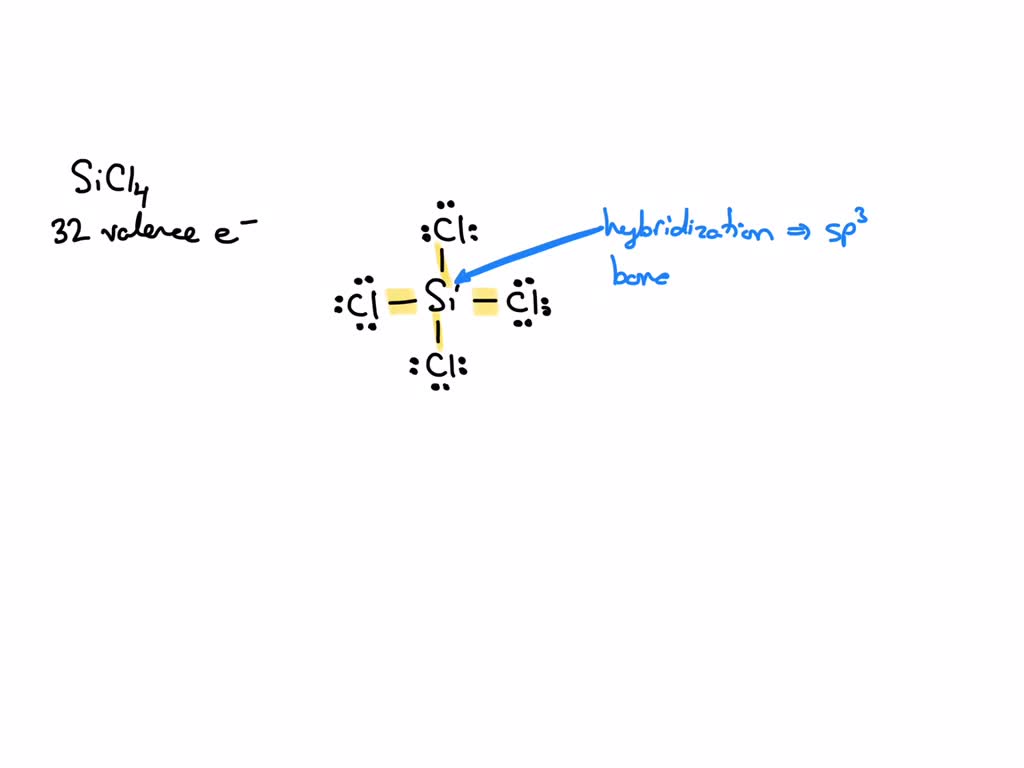
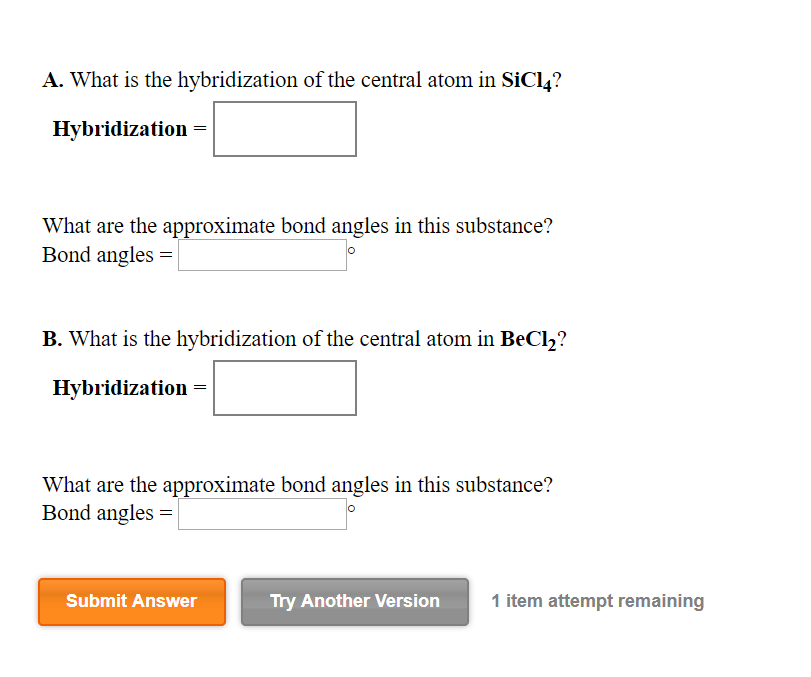
SOLVED: What is the hybridization of the central atom in SiCl4? Hybridization = What are the approximate bond angles in this substance? Bond angles = fill in the blank 2° B. What
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than
Hybridization is a phenomenon that takes place in an atom before chemical bonding. How is hybridization responsible for the observed structure of SiCl4? - Quora

SOLVED: 4a) Using Valence Bond Theory, show the hybridization and bonding scheme for silicon tetrachloride (SiCl4): (a) write the atomic orbital diagram for the central atom, (b) circle the atomic orbitals that
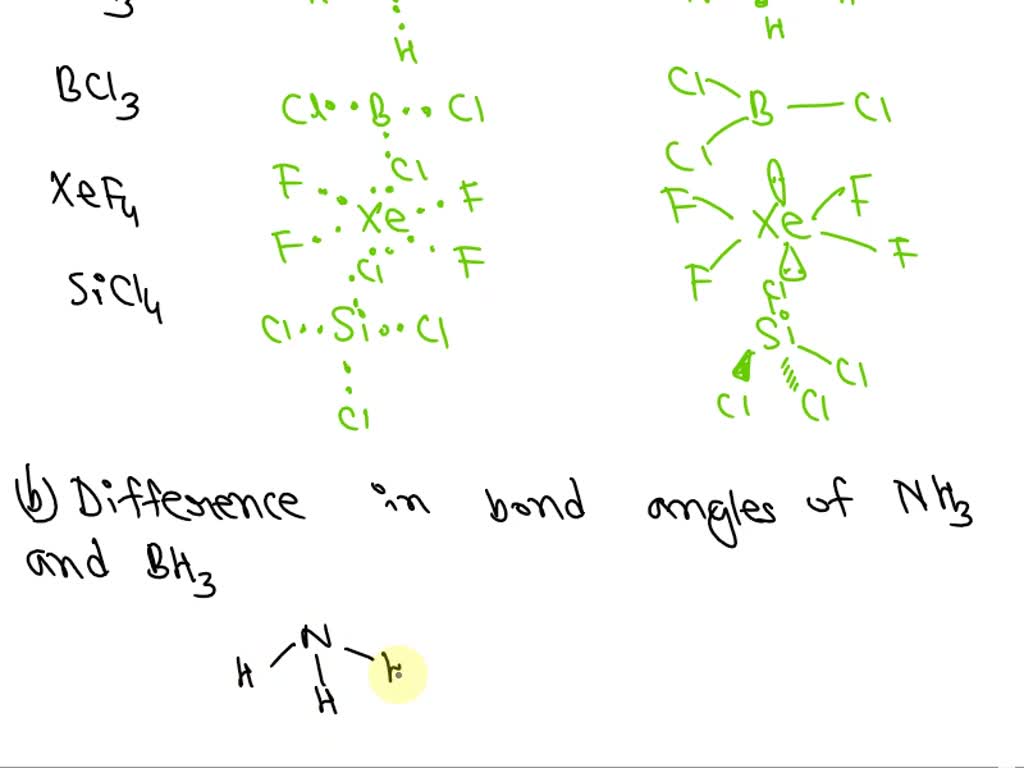
SOLVED: a) Draw the Lewis structures and molecular shapes of NH3, BH3, PCl5, XeF4 and SiCl4, and indicate the hybridization types and geometric shapes. b) Compare the bond angles in NH3 and