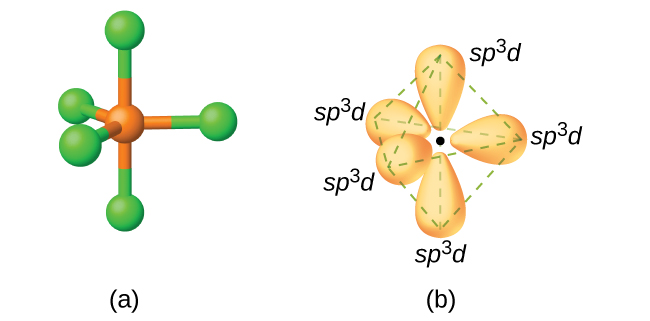
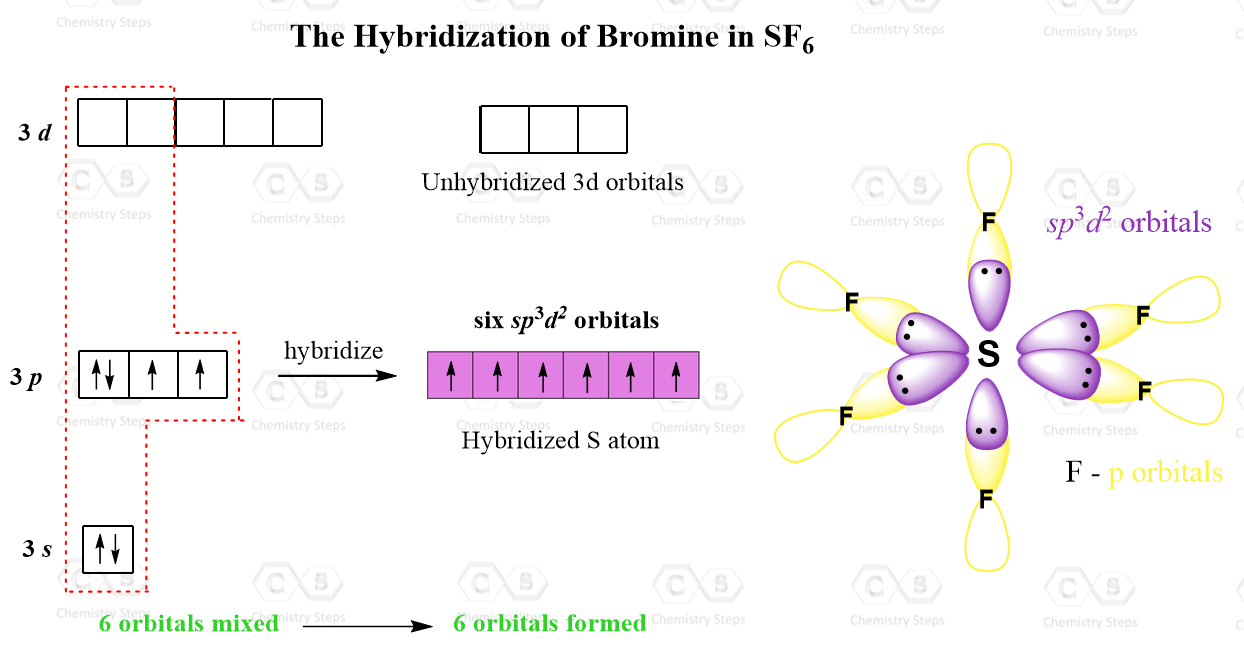
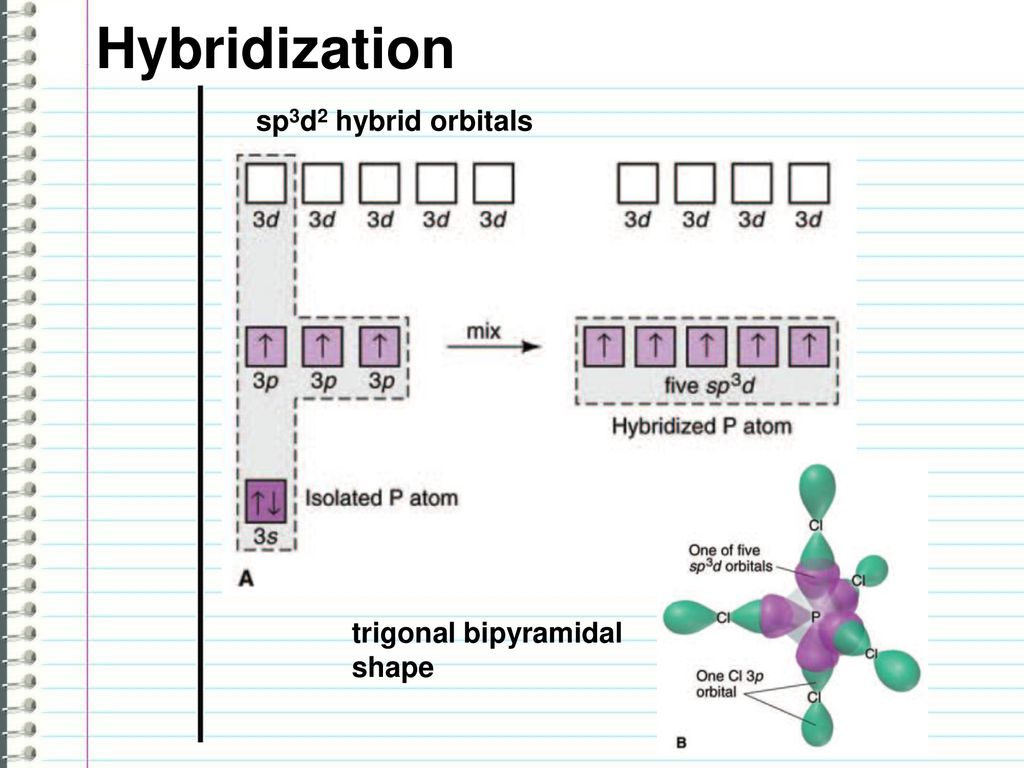
Hybridization || sp3d || sp3d2 || sp3d3 || Formation of PF5, SF6 and IF7 || Chemical Bonding 11th - YouTube

Hybridization || sp3d || sp3d2 || sp3d3 || Formation of PF5, SF6 and IF7 || Chemical Bonding 11th - YouTube

A molecule containing a central atom with sp3d2 hybridization has a(n) ______ electron geometry. a) trigonal planar b) trigonal bipyramidal c) octahedral d) tetrahedral e) trigonal pyramidal | Homework.Study.com

Sp3d2 Hybridization Has 1s 3p And 2d Orbitals That Undergo Intermixing To Form 6 Identical Sp3d2 Hybrid Orbitals Stock Illustration - Download Image Now - iStock
What are the shapes and bond angles of sp, sp2, sp3, sp3d, sp3d2 hybridised orbitals respectively? - Quora


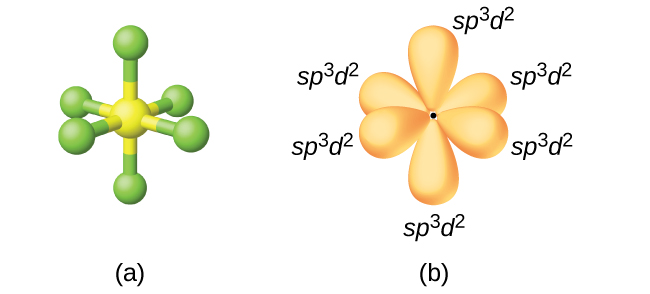
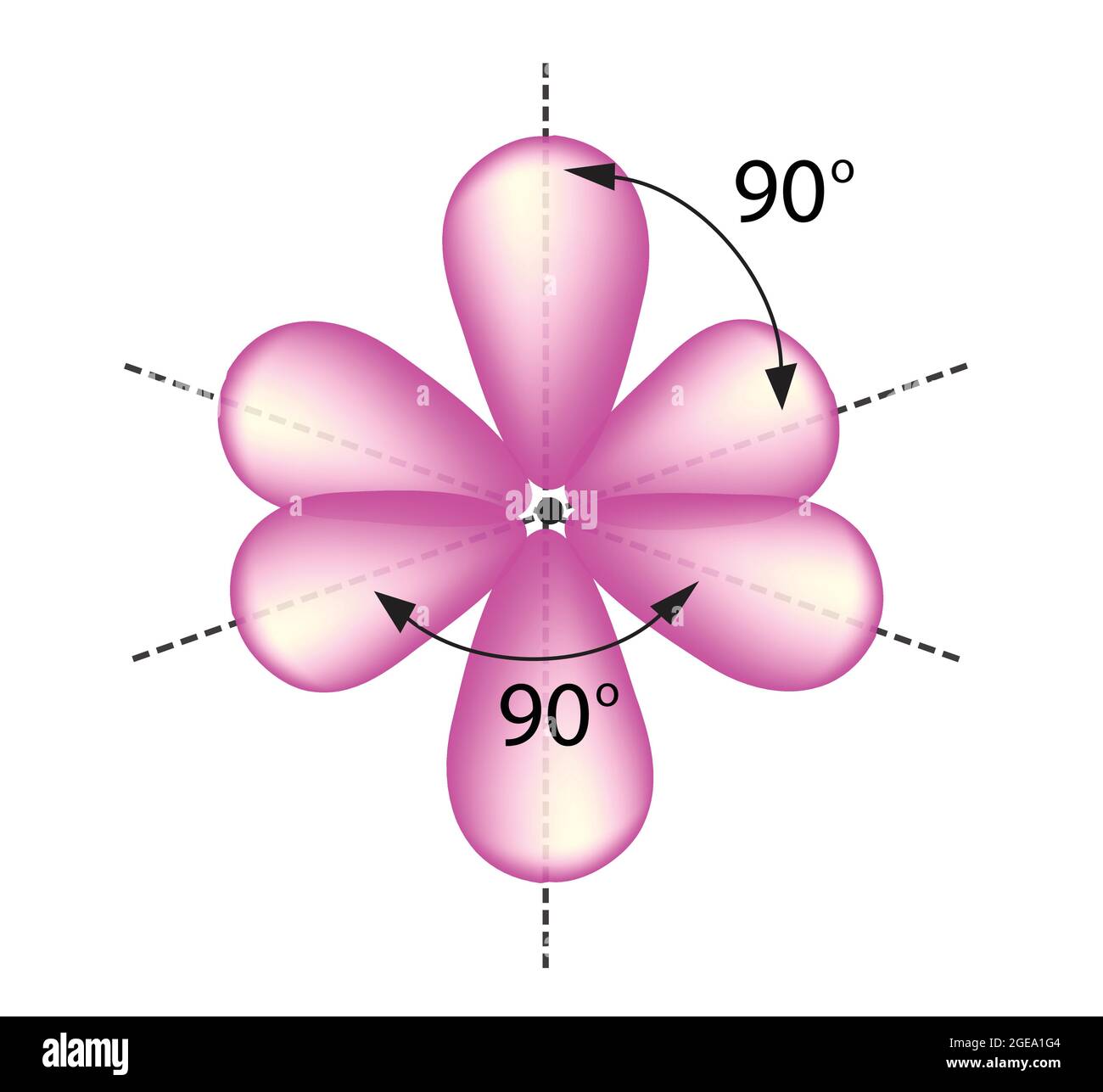

![Sp3d2 Hybridization - Download Free 3D model by AK Chemistry (@AKchemistry) [53efdc3] Sp3d2 Hybridization - Download Free 3D model by AK Chemistry (@AKchemistry) [53efdc3]](https://media.sketchfab.com/models/53efdc35286548f998c8e59f077c2a63/thumbnails/6f6b56c569b0494bbc41bf15c4c68b10/cd3ce7afc92c43a8b38376c034db4bc3.jpeg)
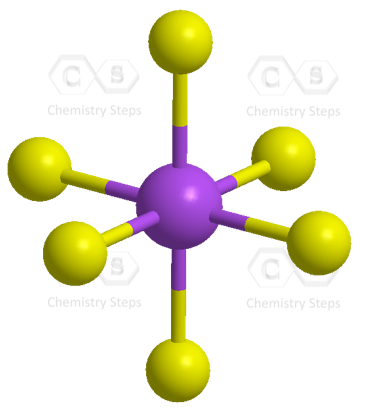
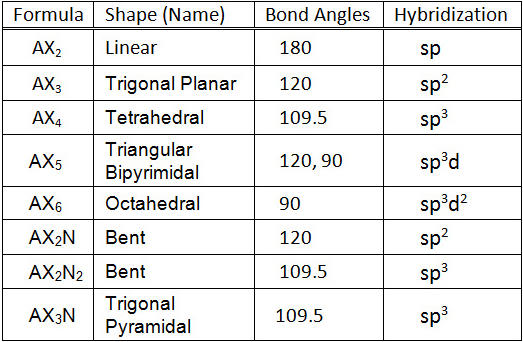
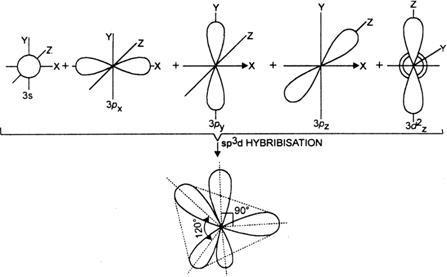

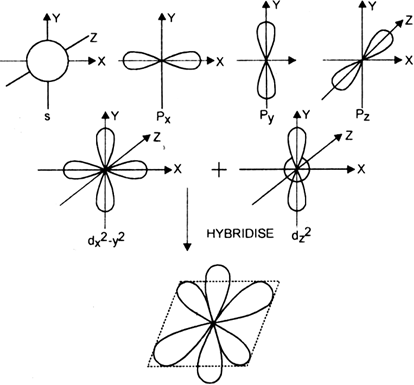
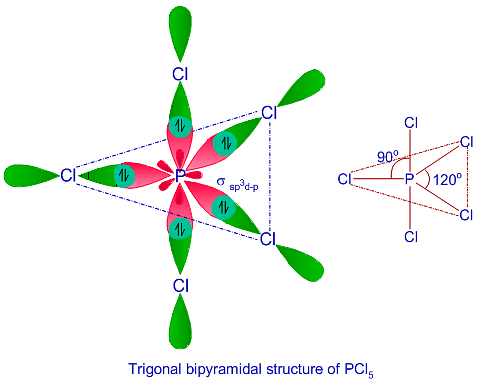


![Solved] Molecule obtained by sp3d2 hybridization has bond angle (s) Solved] Molecule obtained by sp3d2 hybridization has bond angle (s)](https://storage.googleapis.com/tb-img/production/21/08/Reported_23-Aug-2021_Shashi_D8.png)